- 1) Introduction
- 2) Chemistry and Synthesis
- 3) Synthesis Q & A
- 4) GHB Analogs and Derivates
- 5) Precursors
- 6) Physical/Chemical Properties
- 7) Info Resources
- 8) References
1) Introduction
This file deals with the synthesis of GHB and related compounds. It is assumed that the reader already know about the pharmacological aspects of GHB (or else shuld consult the "General Info" references below). It is highly dangerous to attempt a synthesis of GHB without the proper knowledge of practical organic chemistry, and illegal and immoral to even try to sell such a product. This text does not in any way encourage anyone to break the law, or ingest GHB or related pharmaceuticals. Depending on where you live, the manufacture, use and possession of GHB may range from being perfectly legal to being a felony. I suggest you check out your local laws before doing anything stupid. And - please don't mix GHB with alcohol.2) Chemistry and Synthesis
The far most simple way to produce GHB is by the hydrolysis of the corresponding lactone (a cyclic intramolecular ester) to the desired hydroxy acid. Ester hydrolysis can be done in two ways: An acid catalyzed reaction or a base catalyzed reaction. The base catalyzed reaction is our choice here, because the reaction is not reversible like the acid catalyzed one and therefore we will get higher yields, and we will get the sodium salt of GHB, as the free acid is not stable, and will immediately cyclize into gamma-butyrolactone again.
Gamma-Butyrolactone + NaOH => Sodium Gamma-Hydroxy Butyrate (Na-GHB)
Lab procedures for the synthesis of GHB salts:
Please follow common Lab Safety procedures. Wear a lab coat and protective glasses. You will work with hot caustic solutions and solvents! Be aware of the risks associated with the manufacture of GHB! Never work alone!
Sodium GHB
Procedure:
Dissolve 130 grams (3.25 moles) of pure sodium hydroxide in 400ml of tap water in a 1000ml glass container while stirring with a
glass rod or similar. The dissolution is exothermic, and the solution will heat up. When everything has dissolved to form a clear solution,
slowly add 250ml (280 g, 3.25 moles) of gamma-butyrolactone in 50 ml portions with good stirring. The addition of gamma-butyrolactone to
the sodium hydroxide solution is also exothermic, and if it is added too fast the solution will begin to boil, and we don't want that. Keep
track of the temperature with an immersed thermometer. The addition of the gamma-butyrolactone will take somewhere between 20-30 minutes.
When everything has been added, let the mixture react for an additional 10 minutes with occasional stirring.
Now it is time to see if the reaction has gone to completion by checking the pH with universal pH paper. We are aiming for a pH of 7-8. If it is too high (pH > 8), then add 10 ml of gamma-butyrolactone and let react for a few minutes more. If the pH is too low (pH < 7), add a few ml of concentrated NaOH solution. Continue like this until the pH level is within the desired limits.
The solution is perfectly clear and tastes slightly salty. It may be slightly yellow colored, but not much if pure enough butyrolactone was used (distillation of the lactone before use takes care of this problem). If an acid is used to neutralize a too basic a solution (instead of adding more lactone), crystals of the sodium salt of the acid can precipitate in the solution, and the taste is severely impaired. The final solution will be around 750 mL 50% NaGHB. The solution can be concentrated (by boiling off excess water) to ~600mL without it crystallizing at room temp, but if concentrating as far as to ~500 mL it will invariably solidify.
Preparation of Sodium GHB using Sodium Bicarbonate (Baking Soda, NaHCO3)
Written by Chromic
Add 273 g NaHCO3 (3.25 moles) to 1125 mL distilled water in a glass container. Slowly bring the solution to a boil while stirring with a glass rod or similar. All of the baking soda will dissolve. Carbon dioxide will be seen leaving the solution as it comes to a boil. This is the sodium bicarbonate breaking down into a slightly strong base, sodium carbonate:
2 NaHCO3 -> Na2CO3 + H2O + CO2
Reduce the heat to a light boil, and slowly add 250ml gamma-Butyrolactone (280g, 3.25 moles). The addition is not immediately exothermic as with the sodium hydroxide synthesis. Keep this solution at a light boil for 30 minutes. Check the pH with universal pH paper. We are aiming for a pH around 7, but anything 6 to 8 is perfectly safe. If the pH is too high, add a small amount more GBL and continue to reflux.
The solution will be perfectly clear and should be absolutely colorless. If it is not perfectly colorless, i.e. if slightly impure butyrolactone was used and the solution has taken on a light yellow color, add about 100 mL of activated charcoal. Allow this to boil for 10 minutes. Cool the solution then filter, washing the activated charcoal two or three times with 50 ml portions of cold water. 410g of NaGHB will be made in this synthesis. This solution can be concentrated to about 50% NaGHB before it will start to crystallize. If you wish for a powder, heat until the temperature of the solution reaches 150°C then pour onto a flexible metal sheet and allow it to cool and solidify.
This synthesis is perfect for use where there is no ACS, Food or Electronics grade sodium hydroxide available.
Literature methods
Sodium GHB has been made from NaOH and butyrolactone in water[8], in methanol[10,11], and aqueous ethanol[12]
Potassium GHB
Use the ethanol synthesis described above for sodium GHB, but substitute the 130 grams of NaOH for 182 grams of KOH (This calculation is based on the heavier K atom, and the higher water content of KOH versus NaOH). Using KOH gives users of K-GHB that Potassium supplement that is by some said to be needed in connection with administration of GHB. Bear in mind that (powdered) K-GHB is slightly less active (by weight) than Na-GHB as the K ion is heavier than the Na counterpart. Differences between K-GHB and Na-GHB is that the K salt is more soluble in water than the Na salt, and the taste is more like salt/licorice instead of the salt/soap taste of Na-GHB. In the book "Better Sex Through Chemistry" by J. Morgenthaler it is pointed out that "[GHB] has a salty/licorice flavor" and it is obvious that the author tried the K salt.Calcium GHB [5]
74 g analytically pure calcium hydroxide are suspended in 200 ml of tap water. 160 ml 4-butyrolactone are added in portions (each portion about 5 to 10 ml) and under stirring to this suspension at room temperature. After addition of 20 ml the reaction mixture warms to about 50° to 60°C. The addition of 4-butyrolactone is controlled such that the temperature remains between about 50° and 60°C, which takes about 1 hour. During this time the calcium hydroxide has dissolved practically completely. The reaction material is contaminated with a slight rust-yellow precipitate. It is thinned down with 300 ml methanol, is left for four hours to itself and is then filtered through a folded filter. The clear filtrate is cautiously treated with 200 ml acetone in the way that after each portion of acetone causing a precipitate time is allowed for the precipitate to redissolve. A waterclear solution is obtained which is placed for crystallization. After two hours of standing colorless crystals start to deposit. In this state the crystallization is accelerated by continuous addition of acetone (in total 100 ml). The crystallization time is 24 hours. The crystals are sucked off and are washed initially with 50 ml methanol and then additionally with 60 ml acetone. The crystals are dried at temperatures from about 60° to 80°C. in a drying cabinet. Yield: 230 g. Melting point 166-168°C. (immediately). The product is the waterfree nonhygroscopic calcium salt of the 4-hydroxybutyric acid. It is dissolvable as desired in water, the aqueous solution has a pH- value of 7 to 7.5. The salt can be stored as long as desired and does not change in air. Even upon storage no water is attracted from the air.
The residue crystallizes to a mass of colorless crystals, which is after dried at temperatures from about 60° to 80°C. Yield: about 105 g. Melting point 164-166°C. The product is Di-(4-hydroxybutyric) calcium. It is recrystallized by dissolving in little methanol followed by adding of acetone to cloudiness, and crystallizing in the cold.
Instead of methanol also ethanol and isopropanol can be employed for recrystallization with the same success. Without employing water containing alcohols as recrystallization medium or as additive of the recrystallization and purification no stable and in particular no nonhygroscopic calcium salts are obtained. The water content of the alcohols should be from about 3-10% by volume. The such obtained final product does easily dissolve in water, is not hydroscopic and has a pleasant aromatic odor.
Magnesium GHB [5]
60 g magnesium hydroxide (analytical grade) are suspended in 200 ml tap water under stirring. In a stream and under stirring 160 ml butyrolactone are mixed into this suspension. Then the mixture is heated on a water bath for 6 hours under stirring in a 2-liter-flask. The magnesium hydroxide dissolves practically completely. The flask is allowed to stand overnight, while contaminants deposit and the solution is decanted without effort from the contaminant deposit. The water clear decantate is initially stirred with 100 ml acetone for 10 minutes. The colorless sirupy liquid, which now turned more viscous, is mixed again with 100 ml acetone as described above, the acetone is again removed by decanting and the fairly viscous, colorless sirup is left to itself at room temperature for about 2 to 4 hours. It solidifies to a colorless crystal mass, which is comminuted in a mortar and dried for several hours in air. Melting point 76°C to 78°C. Yield: 314 g in analytically pure form.
This magnesium salt contains about 5 mole of water of hydration. It is not hydroscopic, is stable and can be stored for arbitrary long times. By drying over several hours at 40° to 50°C it loses part of its water (1 mole) of crystallization and then melts at 118° to 120°C. Waterfree magnesium 4-hydroxybutyrate can be produced by removal of water by sublimation and/or evaporation of water under decreased partial pressure of water and at elevated temperature or by crystallization from a solution containing an organic solvent. The waterfree salt melts at 172-174°C. The chemical analysis shows 10.50 weight percent magnesium (calculated 10.55 weight percent magnesium). All modifications are nonhygroscopic and stable during storage. 1g of the magnesium salt dissolves in 2 ml water at room temperature, the pH of the aqueous solution is 7.
It dissolves easily in water, methanol and ethanol, it does not dissolve in ether and hydrocarbons, it is not hygroscopic, is storable and has a pleasant aromatic odor.
Other salts
The Lithium and Ammonium salts of GHB would be dangerous to ingest. Lithium ion is toxic, and together with NH3 lactone becomes pyrrolidone.
3) Synthesis Q&A
Q: Can I use lye instead of pure sodium hydroxide?A: No, that could have unpredictable results on your health. Hardware store lye does not have anywhere near the rigorous purity criterions of for example food grade, ACS grade or electronics grade. Some people tells about successful stories using lye, which really is possible, but as said, the results are unpredictable.
Q: I do not have the glassware you say are needed, can I boil the solution in a pot on the stove instead?
A: No, you can not. The sodium hydroxide will corrode the metal, and assorted metal ions will get into your product. You can of course use simpler glassware than in my suggestions, and make the neccessary adjustments of the procedure.
Q: I cannot recrystallize the Na-GHB from ethanol. It forms a sticky mess.
A: Your Na-GHB is not dry, or your ethanol is not anhydrous. Water makes the recrystallation almost impossible. The fact that the sodium GHB is deliquescent (hygroscopic) does not make this better. You must dry the GHB thoroughly, preferably in a vacuum desiccator before attempting recrystallization, or any other improvised alternative. The ethanol you are planning to use (most often supplied in a purity of 95%, the rest being water) must be dried by drying over anhydrous calcium sulfate followed by distillation from calcium oxide with adequate measures taken to exclude moisture from the reaction.
Q: Where can I buy butyrolactone/Is it safe to buy butyrolactone?
A: I have no idea how the situation is for you in your country. The answers to these questions highly depends on who you are and where you live. However, you can contact any of the many sellers of GHB Kits which can be found online.
Q: Is GHB legal to manufacture/sell/possess etc?
A: As above, this depends on where you live. However, where GHB yet isn't scheduled, GHB shuld be legal to both possess and manufacture (don't take my word for it!). Erowid has a good page about the legal aspects of GHB.
4) GHB Analogs and Derivates
Attempts have been made to prolong and/or slightly modify the effects of GHB, with small alterations of the GHB molecule. This section is also known as GIHKAL (GHB-analogs I Have Known And Loved :-)
- Methyl 4-Acetoxy Butanoate (MAB)
- gamma-Butyrolactone (GBL)
- 1,4-Butanediol (1,4-BD)
- 1,4-Butanediol Diacetate (BDDA)
- trans-4-Hydroxy-Crotonic Acid (T-HCA)
- gamma-Hydroxy-Valeric Acid (GHV)
Methyl 4-Acetoxy Butanoate (MAB) [14,15]
Dosage: 250-2500mgDuration: 6-12 h (depending on dosage)
Qualitative effects: The effect of this ester is mostly a powerful muscle relaxant effect, with a very long duration. The accompanying high is less euphoric than the one produced by GHB, although the somatic effects are very nice. Another bioassay can be read here.
Comment: The synthesis below can also be used to make EAB (Ethyl 4-Acetoxy Butanate), if you substitute absolute ethanol for the methanol. The small amount of methanol released over time in the metabolism of MAB is probably not harmful, and the toxicity of EAB is in the literature said to be in the same ballpark as GHB. The smell and taste of MAB is much like petroleum ether.
Synthesis: 80 grams (71.5ml) of gamma-Butyrolactone and 8 grams (4ml) 98% H2SO4 was dissolved in 500ml of methanol in a 1L erlenmeyer flask, which was left standing for a week with occasional swirling. Powdered sodium carbonate was carefully added until further additions did not produce foaming, then 20 grams anhydrous sodium sulfate was added to dry the solution. The solution was filtered and the excess solvent was removed by distilling up to 75°C, discarding the distillate. The concentrated residue was taken up in 250 ml of water and was extracted with 3x200ml CHCl3. The extracts was dried over MgSO4, and filtered with suction. The solvent was removed by distillation up to 80°C (save this impure chloroform, dry it over MgSO4 and re-distill it, saving the fraction between 60-62°C for reuse). The residue was fainty yellow, and was slowly poured into a 1000ml round-bottomed flask containing 80g acetic anhydride and 100ml pyridine, which was clamped in an ice-bath. After the addition was complete, the stoppered flask was left overnight with the ice allowed to melt. A solution of 100ml concentrated hydrochloric acid (12M), 100 ml water and 200g of ice was prepared and carefully added. Two layers formed, and 100 ml chloroform was added. The aqueous layer was extracted with three 100 ml portions of chloroform, and the nonaqueous layer was combined with the extracts. The extract was carefully treated with 50ml saturated aqueous sodium bicarbonate in a separatory funnel (a lot of CO2 gas was evolved), followed by shaking, and the aqueous layer was removed. The organic layer was then washed with 50 ml saturated sodium chloride solution, then dried over anhydrous magnesium sulfate, and filtered. The dried solution was freed from chloroform by distilling up to 100°C (save this impure chloroform, and purify as above). The residue was a slightly yellow liquid amounting about 60ml. This was distilled at the water pump, discarding the fore-run consisting of mostly pyridine. The ester came over at 97-107°C, and was clear, water-white and had a high index of refraction. Yield about 35 grams of pure methyl 4-acetoxybutanoate.
gamma-Butyrolactone (GBL)
Dosage: 0.5-3mlDuration: 2-5 h
Qualitative effects: Very similar to GHB. More muscle relaxing and less prone to give clonic muscle movements than GHB. Slightly longer acting, and has a more sedative feeling than GHB. May easily give gastrointestinal disturbances like nausea, diarrhoea and gas.
Comment: It is not clear if ingesting butyrolactone is toxic or not. It is not cancerogenic as some sources say, but is probably not good for you. The taste is extremely disagreeable. The compound has been used as a GHB pro-drug in some studies, for example by GHB discoverer H. Laborit [9]. Sold in disguise as the GHB substitute Renewtrient.
1,4-Butanediol (1,4-BD)
Dosage: 0.5-3mlDuration: 2-5h
Qualitative effects:
Comment: This compound is marketed as the active ingredient in the "natural product" Borametz, sold for the purpose of promoting Growth Hormone release.
Synthesis: Available commercially from a number of sources.
1,4-Butanediol Diacetate (BDDA)
Dosage: 1-3ml
Duration: 2-5h
Qualitative effects: (as repported by Methaco(s)mic) I have only used doses between 1.0 and 1.5ml. 1.0ml is not felt much and 1.5ml is like a medium dose of GHB. Ordinary 1,4-butanediol feels to me almost exactly as GHB, so what does acetylation do to the effects? It can be felt after 5-10 minutes (tastes like gasoline) the peak of effects occur at the 40 minutes point and then the intoxication gradually dissipates during the following hours and at the 3 hour point the intoxication is over. Perhaps a weak sedation and muscle relaxation lasts to the 5-hour point. The effects are GHB like but not as nice and not euphoric, nothing sexual and the disinhibition is minimal. Relaxing and anxiolytic properties, but not fun. Rather much like MAB perhaps, but without the nice long-lasting positive effects. My stomach doesn't like this stuff I feel like I have to burp but I can't.
Comment: The smell is very nice and flowery, but taste is unpleasant and gasoline-like. The water solubility is very low.
Synthesis: 20ml 99% 1,4-butanediol and 45ml 98% acetic anhydride was mixed in a 100ml round-bottomed flask and boiled under reflux for 30 min. The mixture was poured into 200ml water and extracted with 2x70ml chloroform, the pooled chloroform extracts washed with 2x50ml saturated sodium carbonate solution, the organic layer dried over anhydrous magnesium sulfate, filtered and the chloroform removed by distillation. The residue was then distilled with aspirator vacuum to yield 25ml DABD.
trans-4-Hydroxy-Crotonic Acid (T-HCA) [13,19]
Dosage:
Duration:
Qualitative effects:
Comment: T-HCA is 16% more potent as a GHB receptor agonist than GHB itself, and most T-HCA (trans-4-hydroxy-2-butenoic acid)
derivatives are more active than the corresponding GHB homologs. The 4-CH3 analog is 9%, and the 4-Ph analog is 27% more potent than GHB
itself. The 4-C6H11 analog is 16% less potent than GHB, and cis-4-Hydroxy-Crotonic Acid (C-HCA) is inactive. T-HCA has also been
identified as a naturally occurring substance in the CNS, which dismisses the theory of T-HCA just being a synthetic semi-rigid analog of
GHB, but as a possible endogenous receptor ligand, which also competes at GHB receptors, and possibly possesses specific functions of its
own.
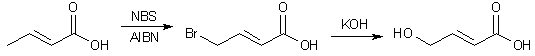
To a solution of 20g (0.23 mole) of crotonic acid (2-butenoic acid) in 200ml of dry benzene, 45.6g (0.25 mole) of N-bromosuccinimide was added under nitrogen. The solution was brought to a gentle reflux with stirring and was treated with 0.5g (3.7 mmol) of 2,2'-azobisisobutyronitrile as a radical initiator. Refluxing was continued for 2 h, and the solution was cooled to 10°C. The resulting white precipitate was filtered off, and the filtrate was evaporated in vacuo. The residue was taken up with 200ml of carbon tetrachloride and the mixture was cooled to 0°C and filtered. The filtrate was evaporated in vacuo to give 38 gram of a mixture consisting of 85% 4-bromocrotonic acid and the rest unreacted starting material. Pure 4-bromocrotonic acid can be obtained by multiple recrystallizations from petroleum ether.
To a cold solution of 12g (72 mmol) of 4-bromocrotonic acid in 120 ml of water was added dropwise 240 ml of a 2M KOH solution in water. After the addition was completed, the solution was successively heated under reflux for 5 minutes (oil bath temp 120°C), cooled in an ice bath, and acidified with dilute H2SO4. The medium was evaporated under vacuum, and extracted with ethyl ether. After the drying and evaporation of the solvent, the residue was chromatographed on a silica gel column eluted with a mixture of EtOAc:MeOH (97:3) to yield 5.22 g (71%) of pure T-HCA. After recrystallization from EtOAc, the mp was found to be 108°C.
gamma-Hydroxy-Valeric Acid (GHV, 4-Methyl-GHB) [13]
Dosage: Similar to GHB.
Duration: Similar to GHB.
Qualitative effects: See my 4-Methyl-GHB Document
Comment: According to reference [13], GHV is 15% more potent as a GHB receptor agonist compared to GHB itself, and nearly as potent as T-HCA
(trans-Hydroxy Crotonic Acid). The 3-methyl derivative of GHB (GHV is the 4-methyl derivative) is 7% more potent, and the 4-phenyl derivative is just slightly more potent
than GHB itself.
Synthesis: 8 grams (0.2 moles) of sodium hydroxide was dissolved in 50 ml of methanol with gentle heating, and some insolubles was filtered off. 20 grams of gamma-valerolactone (0.2 moles) was added as fast as the exothermic reaction allowed, and the solvent was evaporated in vacuo and the wet, soapy residue was dried in a desiccator over CaCl2. The crude product was finely ground in a mortar, placed in a buchner funnel, washed with 200 ml of acetone and sucked as dry as possible at the pump. After drying over CaCl2, the Sodium gamma-Hydroxy-Valerate, a deliquescent, white, crispy and slightly soapy powder, weighed 26.9 grams (96% of theory) and had a pleasant aromatic odor, and the taste was not unlike that of Sodium GHB itself.
delta-Hydroxy-Valeric Acid (DHV) [13]
Dosage: Similar to GHB.Duration: Similar to GHB.
Qualitative effects: Similar to 4-Methyl-GHB
Comment: Using delta-Valerolactone as starting material yields Sodium delta-Hydroxy-Valerate (DHV, 5-Hydroxy-Valerate), which is just slightly less potent than GHB [13].
Synthesis: Same as for 4-Methyl-GHB
5) Precursors
The obvious precursor for the synthesis of GHB is gamma-Butyrolactone. It can be made from pre-precursors such as Tetrahydrofuran (THF) with oxidants such as Ruthenium tetroxide[2], calcium hypochlorite[3] and nitric acid[4].
4-Halo-butyric acid derivatives (chloro, bromo, iodo) can also be used. As
in the synthesis below, they can be converted to gamma-butyrolactone by
distillation with sodium methoxide.
gamma-Butyrolactone from 4-bromobutyric acid: To a solution of 7.8 g of sodium in 500 cc of absolute alcohol was added 60.5 g of 4-bromobutyric acid. The reaction mixture was boiled under a reflux condenser for about five hours. During this time sodium bromide separated. The alcohol was distilled from a steam bath, and the lactone was separated from the sodium bromide by extraction with ether. The ether was evaporated and the lactone distilled under ordinary pressure. The yield was 21.2 gram (67%) of product boiling at 202-206°C.
An alternative may be free radical chlorination of butyric acid with sulfuryl chloride in the presence of peroxides[6], and separate the isomers through distillation, make the sodium salt of 4-chlorobutyric acid, and cyclize to the lactone as with the 4-bromo derivative above.
gamma-Butyrolactone can also be made from 4-methoxybutyric acid[7], 3-phenoxypropylcyanide[8], gamma-diethylaminobutyric acid[1] and beta-chloro ethyl vinyl ether[18] as well as many other a bit too exotic chemicals. Industrially, it is commonly made by reacting acetylene with formaldehyde under high temperatures and pressures.
A very unusable synthesis comes from Journal of Chemical Education [20]:
A five-membered cyclic ester, gamma-Butyrolactone, was prepared from GHB
using a microscale reflux method. Cyclization yielded a product with a boiling
point significantly greater (by 129 °C) than that of the open-chain analogue.
Purification of Precursors
CA 54; 4393i
Outlines of a purification of gamma-butyrolactone.
US Pat No 4,851,085
Purification of gamma-butyrolactone to remove metal ions and color-forming impurities.
US Pat No 3,891,511
Multi-stage purification of 1,4-butanediol
Other Texts Worth Looking Up
US Pat No 4,983,632
Alcoholism treatment with GHB preparations and pharmaceutical compositions designed to hide the taste of GHB.
CA 59; 11234e
Sketchy synthesis of GHB and GABA and some pharmacological data.
US Pat. 5,380,937
Synthesis of salts and amides of GHB with improved pharmacological properties.
6) Physical/Chemical Properties
gamma-Butyrolactone
Mol wt 86.09; mp -43.53°C; bp 204°C; d 1.12 g/mlCAS No: [96-48-0] Flash point: 98°C
Miscible with water, soluble in methanol, ethanol, acetone, ether, benzene
LD50: 1720 mg/kg (orally, mouse) 1540 mg/kg (orally, rat)
Uses: Solvent, paint remover, capacitor electrolyte, in organic chemistry
Synonyms: GBL, BLO, butyrolactone, gamma-hydroxy butyric acid lactone, 1,2-butanolide, 1,4-butanolide, 4-butanolide, 2-oxanolone, tetrahydro-2-furanone, dihydro-2(3H)-furanone.
Sodium GHB
Mol wt 126.09; mp 145-146°CCAS No: [502-85-2]
LD50:2700mg/kg (orally, rat)
Synonyms: Gamma-OH, sodium oxybate, sodium gamma-oxybutyrate, Somatomax PM, Wy-3478, NSC-84223, Somsanit, Anetamine.
Potassium GHB
Mol wt: 142.20Calcium GHB
Mol wt 246.16; mp 164-166°C, 166-168°CMagnesium GHB
Mol wt 230.39; mp (anhydrous) 172-174°C; tetrahydrate 118-120°C; pentahydrate 76-78°C7) Info Resources
Books and publicationsBetter Sex Through Chemistry Contains a 50-page chapter on the benefits of GHB
GHB: The Natural Mood Enhancer The new book, which should clear all confusion around GHB
Cognitive Nutrition Update: GHB Article from Smart Drug News, Vol 3, No 6 (1994)
General Info Sites
Cognitive Enhancement Research Institute
The Lycaeum GHB Pages
Erowid's GHB Pages
Michael Cohn's GHB FAQ
8) References
[1] R. L. Clarke, Pyrolysis of Some Amino Acids, J. Am, Chem. Soc. 71, 2825-2826 (1949)[2] L.M. Berkowitz Ruthenium Tetroxide as a Multi-purpose Oxidant, J. Am. Chem. Soc. 80, 6682-6684 (1958)
[3] S.O. Nwaukwa, Oxidaton of Alcohols and Ethers using Ca(OCl)2, Tet. Lett. 23(1), 35-38 (1982)
[4] Chem. Abs. 53, 15050
[5] J. Klosa, Nonhygroscopic Salts of 4-Hydroxybutyric Acid US Pat. 4,393,236
[6] M. S. Kharasch, Chlorinations with Sulfuryl Chloride, J. Am. Chem. Soc. 62, 925-929 (1940)
[7] F. F. Blicke, Action of Heat on gamma-alkoxybutyryl Chlorides, J. Am. Chem. Soc. 63, 2488-2490 (1941)
[8] C. S. Marvel, Preparation of the Na Salt of omega-Hydroxybutyric Acid, J. Am. Chem. Soc. 51, 260 (1929)
[9] H. Laborit, Sodium 4-Hydroxybutyrate, Int. J. Neuropharmacol. 3, 433-452 (1964)
[10] Chem. Abs. 107, 6774e
[11] Chem. Abs. 107, 39210w
[12] A. P. Arendaruk, Synthesis of gamma-Hydroxybutyric Acids, Chem. Abs. 59, 11234e (1963)
[13] J-J. Bourguignon, Analogues of gamma-Hydroxybutyric Acid J. Med. Chem. 31(5), 893-897 (1988)
[14] R. Kluger, Treatment of Sleep Disorders US Pat. 4,599,355
[15] R. Kluger, Pharmaceutical Composition and Treatment, US Pat. 4,738,985
[16] G. Koehler, Derivatives of 4-Hydroxybutyric Acid, US Pat. 5,380,937
[17] G. Koehler, Derivatives of 4-Hydroxybutyric Acid, US Pat. 5,753,708
[18] W. L. Nelson, The Preparation of Certain gamma-Lactones, J. Am. Chem. Soc. 52, 3702-3704 (1930)
[19] M. Pinza, Convenient Synthesis of 4-Amino-3-hydroxybutyric Acid J. Pharm. Sci., 67,120 (1978)
[20] Bozak, Convenient Synthesis of gamma-Butyrolactone , J. Chem. Educ. 75-84 (1998)