
INTRODUCTION
This FAQ file was prepared by Matthew Baggott (Matt@Baggott.net) originally for distribution on the newsgroup alt.drugs and was updated for the Drug Dependence Research Center (San Francisco).
- Aug 1, 2003: Erowid : Removed dead link from Wormwood section.
- Mar 17, 2002: Erowid : Updated law section of FAQ to reflect increased availability of Absinthe in Europe and Canada.
- Sep 17, 1997: File last updated by author
Other's Contributions: The following individuals contributed information or editorial skills to this FAQ file: Laurent Hagimont (hagimont@cnam.cnam.fr) and Johnny Svensson (svensson@ISI.edu) supplied information about the current availability of absinthe; Mikael Langbraaten (i5lami@itek.chalmers.se ) provided information on the availability of absinthe in Denmark; Stefan Sandstrom (stefan.sandstrom@mailbox.swipnet.se) and Johnny Svensson also gave information about wormwood's use as a flavoring in alcoholic drinks; William White (bwhite@froggy.frognet.net) provided some interesting food for thought about absinthe's psychoactivity; Dr. Aidan Hampson (aidan@codon.nih.gov) helped clairify the relationship between thujone and THC; Richard Boire (telr@cwnet.com) provided his knowledgable opinions on the legality of absinthe in the United States. Many others have shared anecdotes of their absinthe experiences with me and I am grateful. In addition, though I sometimes disagree with their perspectives, Barnaby Conrad III's beautiful and informative book and Wilfred Arnold's fascinating research provided the inspiration for my own research. All these individuals deserve much credit for helping to compile obscure data. Nonetheless, the perspectives, arguments, and errors of this file are mine alone.
Disclaimer: In a scientific journal, articles are usually reviewed by knowledgable researchers before publication. The reviewers can request changes and decide whether the article is accurate and fit for publication. The World Wide Web is not a scientific journal. The wise reader will recognize this and evaluate information from the Web with caution.
Although this file represents an attempt to discuss absinthe in an even-handed manner, the historical data on absinthe leave much to be desired and it is difficult to make definite conclusions. My opinions on absinthe were formed after substantial research, but they remain just opinions.
I do not recommend or condone absinthe use. Absinthe users of the past sometimes suffered serious toxic effects. Wormwood extract, an ingredient in absinthe, can cause life-threatening problems, including convulsions, kidney failure, and rhabdomyolysis. In other words, it can kill you. This document is not meant to be a source of information on "getting high" nor a source of medical or health advice.
Note on this File: This is a incomplete version of the updated Absinthe FAQ. Some sections (such as History, Subjective Effects, and Toxicity) are still being edited and have been temporarily omitted. I look forward to hearing from readers with comments, suggestions, and complaints.
The file contains the following sections:
- Introduction
- Other's Contributions
- Disclaimer
- Note on this File
- Table of Contents
- What is absinthe?
- How was/is absinthe made?
- How was/is absinthe drunk?
- What ingredient(s) in absinthe are psychoactive?
- Definitely ethanol
- Possibly thujone (from the herb wormwood)
- Thujone's structure and name
- Psychoactivity of thujone and wormwood
- Does thujone act at same site as THC? (probably not)
- Is there sufficient thujone in absinthe to have any effect (probably only if it accumulates)?
- Other occasional ingredients with possible activity (nutmeg, calamus, congeners of ethanol, toxic adulterants)
- What modern alcoholic beverages are related to absinthe?
- What is the legal status of absinthe?
- More information on plants relevant to absinthe
- Wormwood (Artemisia absinthium)
- Other plants containing thujone
- Table on occurrence of thujone in essential oils of various plants
- Van Gogh and absinthe
- Other artists and absinthe
- References and further sources of information
WHAT IS ABSINTHE?
Absinthe is strong alcoholic liqueur made with an herbal extract including wormwood (Artemisia absinthium). It is an emerald green drink (due to the presence of chlorophyll) which is very bitter (due to the presence of absinthin, which has a bitterness threshold of 1:70,000) and is therefore traditionally diluted with cold water which is poured over a perforated spoonful of sugar into a glass containing a shot of absinthe. The drink then turns into an opaque white as the essential oils precipitate out of the alcoholic solution, forming a colloidal suspension. Absinthe was once popular among artists and writers and was used by Van Gogh, Baudelaire, and Verlaine, to name a few. It appears to have been believed to stimulate creativity and to act as a curative and aphrodesiac.
The 1850's saw the beginnings of concern about the results of chronic absinthe use. Chronic use was believed to produce a syndrome, called absinthism, which was characterized by addiction, epileptic attacks, delirium, and hallucinations. Concern over the health effects of absinthe was amplified by the prevailing belief in Lamarckian theories of heredity. In other words, it was believed that any traits acquired by absinthists would be passed on to their children (Murphy and Schneider 1992).
In addition to its effects in heavy drinkers, there were several social reasons why absinthe was ultimately banned. Absinthe's popularity seems to have been part of a general increase in alcohol consumption, particularly in the form of distilled liqueurs. This was accompanied by the beginnings of the awareness of alcoholism as a problem in France. Since wine was considered a healthy drink and absinthe was the most popular liqueur of its time, absinthe was blamed for many alcohol-related problems and became the main target of early prohibition efforts in France. Absinthe's association with the bohemian lifestyle may have worked to compound fears about its effects, much as has happened with marijuana in the United States. In retrospect, absinthe seems to have become the focus of fears about the changes that came with industrialization. Absinthe was subsequently banned in many countries in the early 1900's.
In addition to the many social and political factors which contributed to anti-absinthe sentiment, extensive research documented absinthe's potential for toxicity. From a modern perspective, this research appears poorly designed and limited. Nonetheless, it is clear that absinthe had toxic effects when consumed with sufficient quantities and regularity. It is highly plausible that thujone and related terpenes played an important role in this toxicity, but there are also other possible sources of toxicity. When used in sufficient quantities, ethanol has profound toxic effects. If it is likely that absinthe was toxic to heavy users, it is less clear that the liqueur was uniquely psychoactive. Until more conclusive research is carried out, theories of absinthe's special psychoactivity remain interesting speculation and anecdotes.
HOW WAS/IS ABSINTHE MADE?
There were two general ways in which absinthe was made. The first method, which was more traditional, is described in some detail below. This was the method used by more established and larger absinthe producers. The second method involved flavouring industrially produced (and often impure) ethanol with essential oils extracted from the plants listed below. This second method probably came into practice later and seems to have been used mainly by smaller manufacturers.
Simon and Schulter's Guide to Herbs and Spices tells us that Henri-Louis Pernod used aniseed, fennel, hyssop, and lemonbalm along with lesser amounts of angelica, star anise, dittany, juniper, nutmeg, and veronica. These ingredients were mascerated together with wormwood plants. After leaving the mixture to sit, water was added and the mixture was distilled. Dried herbs, including more wormwood, were added to the distillate, which was then diluted with alcohol to give a concentration of about 75% alcohol by volume (Simonetti 1990). Different absinthe manufacturers used slightly different ingredients, sometimes using nutmeg and calamus, both of which have been purported to have psychoactive effects.
A more detailed recipe for the first method can be found in Arnold's Scientific American article:
An 1855 recipe from Pontarlier, France, gives the following instructions for making absinthe: Macerate 2.5 kilograms of dried wormwood, 5 kilograms of anise and 5 kilograms of fennel in 95 liters of 85 percent ethanol by volume. Let the mixture steep for at least 12 hours in the pot of a double boiler. Add 45 liters of water and apply heat; collect 95 liters of distillate. To 40 liters of the distillate, add 1 kilogram of Roman wormwood, 1 kilogram of hyssop and 500 grams of lemon balm, all of which have been dried and finely divided. Extract at a moderate temperature, then siphon off the liquor, filter, and reunite it with the remaining 55 liters of distillate. Dilute with water to produce approximately 100 liters of absinthe with a final alcohol concentration of 74 percent by volume (Arnold 1989).
In addition to these ingredients, manufacturers sometimes added other ingredients to produce the drink's emerald green color. Normally, this color was due to the presence of chlorophyll from the plants. However, in the event that the product was not properly colored, absinthe makers were known to add things like copper sulfate, cupric acetate indigo, turmeric, and aniline green. Antimony trichloride was also used to help the drink become cloudy when added to water (Arnold 1989, 1988). Undoubtedly, some of the toxic effects attributed to absinthe were due to these adulterants.
HOW WAS/IS ABSINTHE DRUNK?
 Although absinthe was sometimes drunk straight or in a variety of mixed
drinks, the classic method of drinking it involves pouring cold water over
a slotted spoon which contains sugar into a glass containing a shot of
absinthe. As the water hits the absinthe, the oils precipitate out, and
the drink changes from a clear emerald colour to an opaque, milky white.
Although absinthe was sometimes drunk straight or in a variety of mixed
drinks, the classic method of drinking it involves pouring cold water over
a slotted spoon which contains sugar into a glass containing a shot of
absinthe. As the water hits the absinthe, the oils precipitate out, and
the drink changes from a clear emerald colour to an opaque, milky white.
There were several types of specialized absinthe drinking paraphenalia. The most famous one is the slotted spoon (example pictured to the right). These spoons' holes served to allow the absinthe to better carry the sugar into the glass. In addition, the spoons were often designed to fit over the glass, balancing on their own.
Heilig, quoted in Lanier (1995), nicely captures the absinthe culture at its peak:
[In Paris] the noon hour is a little fête, when people try to forget that they are working for their living. Master and man go off their different ways... All thoughts of business are put aside for a good hour and a half, or two hours even ... from 11 to 1 or noon. They do not go immediately to eating. They sit outside upon the sidewalk, even in the winter time, look at passers-by and sip their drink. The drink is absinthe. They drink it very slowly; by slow degrees they feel their poor, tired backbones strengthen and their brains grow clearer, and they feel a touch of happiness. It is so pleasant to sit looking at the street and all the pretty ladies passing by. At great cafes, upon frequented boulevards the price is only 10 cents. In the quarters of the working men, you may have absinthe for three cents. The proper thing is to take but one glass. In quantity this is about a Madeira glass of the green drink. Poured into your goblet by the waiter, it does not seem much. You fill the goblet up with water, watch it turn a milky sage tint, with the glittering opal tints one learns to love so well; stir up the mixture with you spoon, take one small sip and let it rest. Now that is nice... In summer especially when the ice water is so agreeable, absinthe captivates the palate by its peculiar and really exquiste fragrance. This fragrance, which is that of paregoric, grows upon you (Helig 1894).
A variation of the traditional drinking ritual is apparently used in Prague where absinthe is currently available. In this variation, a heaping teaspoon of sugar is briefly wet in the glass of pure absinthe, then lit on fire and held over the glass. As the alcohol burns off, the sugar melts into the glass. When the fire gets low, the remaining sugar is stirred into the drink and the drink is quickly drunk. Obviously, this is a method for drinking quickly rather than savoring absinthe's taste.
WHAT INGREDIENT(S) IN ABSINTHE ARE PSYCHOACTIVE?
ETHANOL
Ethanol (normal drinking alcohol) is definitely one main active component. Undiluted absinthe was anywhere from 60% to 85% ethanol. Although some, such as Alfred Jarry, were known to have drunk it straight, it was usually substantially diluted. Still, dilution could not change the fact that absinthe contained a lot of ethanol in comparison to its other ingredients.
THUJONE
Another candidate is the monoterpene, thujone, which is considered a psychoactive convulsant. The sources of thujone in absinthe are the herbs wormwood (Artemisia absinthium) and Roman wormwood (Artemisia pontica). There is good evidence that both thujone and wormwood have psychoactive properties. Some have suggested that this effect is due to thujone binding at the cannabinoid receptor, at which the active components in marijuana act (delCastillo et al 1974). This seems unlikely. Furthermore, it is not even clear that thujone is present in sufficient quantities to play a role in absinthe intoxication. However, it is possible that thujone accumulates in the body and plays a role in the psychoactivity and toxicity of chronic absinthe use.
Thujone is named after the plant from which it was first extracted,
thuja (Thuja occidentalis). Since thujone was also extracted from
other plants before its structure was identified, it is also known as absinthol,
tanacetone, and salviol. According to IUPAC (International Union of Pure
and Applied Chemists) nomenclature, it is officially called 3 thujamone
or 3 sabinone (Albert-Puleo 1978). There are two stereoisomers of thujone:
(-)-3-isothujone (or  - or l-thujone) and
(+)-3-thujone (or
- or l-thujone) and
(+)-3-thujone (or 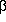 - or d-thujone).
Thujone is the major component of wormwood oil and accounts for up to 90%
of the oil's weight (Simonsen 1949).
- or d-thujone).
Thujone is the major component of wormwood oil and accounts for up to 90%
of the oil's weight (Simonsen 1949).
There have been several reports that wormwood has psychoactive effects. In his excellent book, Pharmacotheon, J. Ott writes that he tried smoking dried wormwood leaves and found it had a definite psychoactive effect (Ott 1993). Pendell (1994) repeated this experiment with similar effects. Furthermore, various other species of the Artemisia genus have been smoked and used as intoxicants in other cultures. Artemisia nilagirica is reportedly smoked in West Bengal for its psychoactive effects (Pal and Jain 1989). Similarly, Artemisia caruthii is inhaled by the Zuni as an analgesic (Ott 1993). However, these experiments yield little insight into the active component(s) of wormwood and whether these components play a role in absinthe's effects. For example, despite being smoked for its psychoactive effects, an assay of Artemisia nilagirica oil found it contained less than one percent total thujones (Uniyal, Singh, Shah, and Naqvi 1985).
There are also indications that thujone itself is psychoactive. Rice and Wilson (1976) have found that (-)-3-isothujone, the dominant isomer in wormwood oil, has an antinociceptive (pain killing) effect, comparable to codeine, when injected subcutaneously in rats. Because the effect is stereospecific and not elicited by similar compounds, the researchers suggest that (-)-3-isothujone acts at a specific pharmacological site.
Thujone's mechanism of action is unknown. Structural similarities between thujone (in its delta-3,4 enol form) and tetrahydrocannabinol (THC, the active component in marijuana) have led some to hypothesize that both substances have the same site of action in the brain (del Castillo et al 1975). However, Meschler et al. (1997) recently presented evidence that that neither thujone, wormwood, nor HPLC fractions from oil of wormwood bind to the cannabinoid receptor at physiologically relevant concentrations. This finding confirms earlier but less direct evidence that thujone does not act at the cannabinoid receptor (Greenberg, Mellors, and McGowan 1978, Browne and Weissman, 1981, Rice and Wilson, 1976). It would seem that thujone acts through some other, yet unidentified, mechanism.
Although thujone is apparently psychoactive, there isn't much direct evidence to confirm its importance in absinthe intoxication. Absinthe is approximately 75% alcohol. Therefore, alcohol's effects should limit the amount of thujone one can ingest. Quite simply, you can only drink a moderate amount of thujone before you become very drunk from the alcohol. Thujone would have to be active at a very low dose or be present in high quantities in order to have any appreciable effect.
Arguing against thujone's importance, B. Max, in the "This and That" column in Trends in the Pharmacological Sciences, made the following dose calculations:
How much thujone was present in absinthe? Steam distillation of wormwood yields 0.27-0.40% of a bitter, dark-green oil (Guenther 1952) In a typical recipe for absinthe, 2.5 kg of wormwood were used in preparing 100 liters of absinthe (Arnold 1989). Typically, 1.5 oz was consumed (diluted with water) per tipple (Vogt & Montagne 1982). This is equivalent to 4.4 mg wormwood oil per drink, or 2-4 mg thujone. This is far below the level at which acute pharmacological effects are observed. Even chronic administration of 10 mg/kg oral thujone to rats does not alter spontaneous activity or conditioned behavior (Pinto-Scognamiglio 1968). The literature on the pharmacology of thujone is, to put it bluntly, second rate, and conclusions as to its effects have been extrapolated far beyond the experimental base (Max 1990).
Although I agree with his feelings about the literature on thujone's activity, Max may underestimate the possible effects of chronic thujone intake. The reference which he uses to support his claim (Pinto-Scognamiglio 1968) actually did find some indications of an effect from chronic thujone intake. Rats treated with 10 mg/kg/day oral thujone increased their spontaneous activity from 4pm to 8pm (the early part of the nocturnal rats' active period). In addition, a subgroup of 6 slow-learning rats significantly improved (in comparison to controls) their acquisition of a shock avoidance task after 7 days of 10 mg/kg/day oral thujone. However, this effect was not replicable in a larger group of 34 rats. Because these 34 rats were naive to the task and therefore of unknown learning ability, we cannot rule out the possibility that thujone may somehow alter the learning abilities of slow-learning rats but not fast-learners.
The appearance of thujone's effects after chronic administration of an otherwise ineffective dose is consistent with the toxicological data on thujone. In rats, at least, thujone accumulates with regular use. In Margaria's (1963) unpublished rat research (cited in Pinto-Scognamiglio 1967), rats fed 10 mg/kg/day orally accumulated about 5% of the daily dose. On the 38th day of the research, convulsions were evident in the rats. Similarly, in the human data, quoted in the toxicology section below, humans taking thujone-containing essential oils experienced convulsions after taking approximately the same dose of oil for several days without incident (Millet et al 1980).
Thus, there are indications that a large enough dose of thujone will be psychoactive and that thujone can accumulate in one's body. However, this does not prove that accumulated thujone was partially responsible for absinthe's psychoactivity. When chronically exposed to a drug, receptors in the brain often become less sensitive to a drug's effects. The brain of a chronic absinthe user might become tolerant to the slow accumulation of thujone, thus blocking any possible psychoactivity. On the other hand, in some cases, repeated doses of drugs can cause hypersensitivity. It is also possible that this occurred with absinthe drinkers. This is just speculation. The toxic effects of repeated thujone ingestion are more definite.
In summary, thujone seems psychoactive although probably not by acting at the cannabinoid receptor. Small ineffective doses may accumulate in the body to the point of having psychoactive and toxic effects. If this is the case, it validates absinthe's reputation for producing an unusual intoxication. Still, this reputation mostly dates to the beginning of the century or earlier, a time when medicine and science were very different from today. Lacking more recent research on thujone and absinthe, it seems reasonable to take reports of absinthe's uniqueness with skepticism. Thujone may play a role in absinthe, but the evidence is not conclusive. Finally, it should be noted that by focusing on one component of wormwood oil, we ignore the many other poorly characterized compounds in wormwood and absinthe's other herbal ingredients which may play some role in absinthe's intoxicating and toxic effects.
OTHER OCCASIONAL INGREDIENTS WITH POSSIBLE PSYCHOACTIVITY
Calamus (Acorus calamus) and nutmeg (Myristica fragrans) were also sometimes used in making absinthe. Both plants have reputations for being psychoactive. (See Panayotopoulos and Chisholm, 1970; and Shulgin, 1966 for information on nutmeg. See Ott, 1993, for a good brief discussion on calamus' use as a stimulant and sedative). However, it seems unlikely that either plant would have been added in the quantities necessary to produce psychedelic effects. Still, either might have exerted minor effects on absinthe intoxication, since both have been said to have sedative effects in moderate amounts.
It is also worth noting that some of the effects of alcoholic beverages are due to congeners of ethanol. Congener is a general term for small molecules other than ethanol which are found in alcoholic beverages. They include aldehydes, esters, and primary alcohols such as methanol and isoamyl alcohol. The amount of congeners in an alcoholic beverage varies depending on the type of drink. In general, a high concentration of one congener will be accompanied by high concentrations of the others. Congener content is significant because they can act as CNS depressants, mucosal irritants, and produce nausea. With the exceptions of methanol and acetaldehyde, there is little good research on congeners' effects. However, they appear to increase the duration of intoxication, the amount of hangover, and the toxicity of alcoholic beverages (Kissin 1974, Murphree 1971). Congeners may be particularly significant with respect to absinthe since the rise of absinthe's popularity coincides with the introduction of alcool d'industrie (made from beets and cereals rather than fruits) into the alcohol industry. Such alcohol would have required secondary distillation to further remove congeners. Since absinthe's strong flavor would have masked the taste of any congeners, it seems possible that the amount of congeners was often quite high and that some less scrupulous manufacturers may have skipped the second distillation.
Absinthe makers sometimes added colorants to achieve the expected emerald color. These adulterants included copper sulfate, cupric acetate indigo, turmeric, and aniline green. Antimony trichloride was also used to help the drink become cloudy when added to water (Arnold 1988, 1989). These ingredients must have contributed to absinthe's toxicity and, perhaps, subjective effects. See the toxicity section for further discussion of these ingredients.
Richard Alan Miller's book The Magical and Ritual Use of Herbs reports that "absinthine (a dimeric guaranolide) is the principal agent in absinthe" and "is listed as a narcotic analgesic in the same group of codeine and dextromethorphan hydrobromide" (p. 52). Although lists of wormwood's constituents (Duke, 1985) contain absinthin (the main source of absinthe's bitter taste), I have found no research which identifies it as having any psychoactivity.
A FINAL NOTE ON ACTIVE INGREDIENTS
In addition to those ingredients mentioned above, there may be other unidentified compounds which are important. We should not overlook the possibility that a group of compounds might together play a central role in absinthe intoxication. Groups of compounds in a plant may have some pharmacological effect which the individual compounds lack when studied separately. (Ginkgo biloba seems to be an example of this).
WHAT MODERN ALCOHOLIC BEVERAGES ARE RELATED TO ABSINTHE?
Herb Sainte and Pernod are names of modern wormwood-free absinthes. Typically, additional star anise is added to balance the flavor. Herb Sainte is manufactured in New Orleans. Pernod is named after Henri-Louis Pernod, who founded the most important absinthe distillery in France in the early 1800s.
Pastis is a similar liqueur to absinthe and was also originally made with wormwood. However, the dominant flavor in pastis is licorice (rather than the star anise of modern Pernod or Herb Sainte). Pastis brands include Ricard, Duval, Jeannot, Casanis, and Henri Bardouin (Steinriede, 1996).
Vermouth, chartreuse, and benedictine all contain small amounts of thujone. In fact, vermouth, which is made using the flower heads from wormwood, takes its name from the German wermuth ("wormwood").
There are, of course, many other essential oil containing drinks, such as Ouzo and Jagermeister.
Wormwood is popular as a flavoring for brannvin (an alcoholic drink made from potatoes) in Sweden.
WHAT IS THE LEGAL STATUS OF ABSINTHE?
(What follows is an attempt to describe absinthe's legal status. The wise reader will remember that I am not a lawyer. There may be relevant laws or legal rulings with which I am unfamiliar.)
Although it is banned in some Western countries, absinthe isn't controlled as a drug but as a food. As with many other things considered poisonous, you aren't allowed to commercially make food or drink containing more than trace amounts of thujone. However, simple possession of thujone-containing ethanol solutions will probably not get you into legal problems. Presumably you would be legally liable for any possible damages if you gave absinthe to others to drink. Artemisia species are completely legal and are attractive perennial ornamental plants.
In the United States of America, absinthe was originally banned by Food Inspection Decision 147 in 1912. Now, thujone is banned as a food additive according to Section 801A of the Federal Food, Drug, and Cosmetic Act of August, 1972. Wormwood was included on a list of unsafe herbs which the FDA released in 1975.
The European Community Codex Committee on Food Additives has restricted the levels of thujone to 0.5 ppm (mg/kg) in food and beverages, 10 ppm (mg/kg) in alcoholic beverages containing more than 25% alcohol, 5 ppm (mg/kg) in weaker alcoholic beverages, and 35 ppm in bitters. Absinthe was banned in Belgium in 1905, in Switzerland in 1907, in Italy in 1913, and in France in 1915.
Absinthe (made with wormwood) is still available in Spain (contrary to Pendell (1995)) and reportedly in Denmark, Andorra, and Portugal as well. It has also recently become popular in the Czech Republic under the brand name "Hill's Absinth."
For more information, see Erowid's Absinthe Law VaultINFORMATION ON PLANTS RELEVANT TO ABSINTHE
WORMWOOD (ARTEMISIA ABSINTHIUM)
 A nice description of wormwood's appearance, biology, and habitats can
be found in the Nature Conservancy's Element
Stewardship Abstract on wormwood. Although the abstract is aimed at
providing information on managing land and plants, it is still worth checking
out.
A nice description of wormwood's appearance, biology, and habitats can
be found in the Nature Conservancy's Element
Stewardship Abstract on wormwood. Although the abstract is aimed at
providing information on managing land and plants, it is still worth checking
out.
WORMWOOD'S CONSTITUENTS
Duke, in the CRC Handbook of Medicinal Herbs gives the constituents of wormwood as:
the essential oil (up to 1.7%) contains phellandrene, pinene, thujone (3 to 12%), thujyl alcohol, thujyl acetate, thujyl isovalerate, bisabolene, thujyl palmitate, camphene, cadinene, nerol, and azulene (chamazulene, 3,6-dihydrochamazulene, 5,6-dihydrochamazulene). Formic and salicyclic acids occur in the saponification lyes of wormwood oil. The herb also contains bitter glucosides absinthin, absinthic acid, anabsinthin, astabsin, artametin, succinic acid together with tannin, resin, starch, malates, and nitrates of potassium and other salts. Lactones include arabsin, artabin, and ketopelenolide (a germacranolide). (Duke 1985, p. 67)
Wormwood oil is produced by steam distillation of the leaves and flowering tops of dried wormwood. In terms of smell, appearance, and flavor, Arctander (1960) describes wormwood oil as:
...a very dark green, brownish-green or bluish green colored liquid with an odor that is intensely herbaceous-green, warm and deep, and a sharp and fresh top note, reminiscent of cedarleaf oil. The body-note is very warm and dry-woody, long lasting and highly interesting as a unique perfume note. The flavor of wormwood oil is intensely bitter, and has an astringent mouthfeel and a long-lasting unpleasant aftertaste. The flavor is pleasant, green-herbaceous, somewhat reminiscent of hop and chamomile only in very high dilution. (Arctander 1960, p. 662)
It is possible to buy wormwood oil from companies that sell essential oils. Caution should be exercised with these oils since they can contain significant amounts of pharmacologically active and/or toxic compounds. Some of these compounds may be absorbed through the skin. If enough essential oil is absorbed or ingested, life-threatening medical problems, including convulsions, kidney failure, and muscle disintegration (rhabdomyolysis), may result.
PHARMACOLOGY OF WORMWOOD
Wormwood has been used medicinally since antiquity. Many of its uses have been supported by modern research. However, for each situation in which wormwood might be useful, there are probably safer herbal and non-herbal alternatives. Wormwood is rarely recommended these days. In fact, the prominent herbalist, Michael Moore, in his list of Herbal-Medical Contraindications categorizes Artemisia absinthium "as lacking any socially redeeming value." As you read on, you may wish to keep this in mind. What follows is neither a source of medical advice nor a guide to self-medication.
As its name implies, wormwood has been used to expel worms from people
and animals. However, Caius and Mhasker (1920) did not find oil of wormwood
to be an effective antihelmintic when tested against the hookworm.
Whatever antiparasitic properties wormwood has may be partially
due to its  -santonin content (Perez-Souto
et al 1992), which is recognized as a medicine for parasitic diseases.
Of course, wormwood's measurable toxicity prevents modern herbalists from
recommending it.
-santonin content (Perez-Souto
et al 1992), which is recognized as a medicine for parasitic diseases.
Of course, wormwood's measurable toxicity prevents modern herbalists from
recommending it.
Wormwood contains unidentified antimalarial substance(s). Alcoholic extracts of the dried leaves have 'considerable antimalarial potential' when administered orally, subcutaneously, or intraperitoneally to mice (Zafar, Hamdard, & Hameed 1990).
Wormwood leaves are used traditionally in Pakistan as an antipyretic (anti-fever) and an active antipyretic compound has been isolated from the dried leaves. This compound alleviates yeast-induced pyrexia in rabbits (Ikramet al 1987).
Dilute (1:1000) oil of wormwood has some antimicrobial activity. Kaul, Nigam and Dhar (1976) found that the dilute oil inhibited the growth of 4 (out of 7) different types of bacteria.
Wormwood is also hepatoprotective (liver protecting). Gilani and Janbaz (1995) found that an aqueous-methanolic extract of Artemisia absinthium protected against acetaminophen and CCl4-induced hepatotoxicity in mice. This protection seems to be at least partially due to inhibition of microsomal drug metabolizing enzymes (MDME), since the plant extract prolonged the sleep-inducing effects of pentobarbital in mice. Gilani and Janbaz speculate that this putative MDME inhibition may be due to sesartemin, which has the methylene-dioxybenzene group common to MDME inhibitors. The presence of antioxidants and calcium-channel blockers in wormwood (Gilani 1994) also probably contribute to its hepatoprotective effects.
For a discussion of wormwood's psychoactivity see the above sections on absinthe's psychoactive ingredients.
OTHER PLANTS CONTAINING THUJONE
According to W. N. Arnold's Scientific American article:
Thujone occurs in a variety of plants, including tansy (Tanacetum vulgare) and sage (Salvia officinalis), as well as in all the trees of the arborvitae group, of which the thuja (Thuja occidentalis), or white cedar, is one. It is also characteristic of most species of Artemisia, a genus within the Compositae, or daisy, family. Wormwood (Artemisia absinthium) and Roman wormwood (Artemisia pontica) were the main sources of the thujone in absinthe (Arnold, 1989, p. XX).
Table II (below) lists the thujone content of many different plants' oils including the Artemisia genus. This list is not exhaustive and I would welcome (referenced) additions. Because the thujone content of plant specimens varies depending on a large number of factors, this table should only be used to give a rough idea of a plant's thujone content. For example, oil from the common culinary herb, sage (Salvia officinalis), is said to contain from 15% - 60% thujone (assayed as 42.5% in the table below). Since sage is GRAS (Generally Recognized As Safe) and widely consumed, its thujone content could be seen as evidence of thujone's relative safety or as evidence of dangerously lax regulation.
TABLE II: OCCURRENCE OF THUJONE IN ESSENTIAL OILS OF VARIOUS PLANTS
|
Plant |
percent |
percent |
percent |
Reference |
|---|---|---|---|---|
|
Artemisia absinthium |
59.9 |
2.3 |
62.2 |
Sacco and Cialva (1988) |
|
Artemisia austiaca |
31.0 |
- |
31.0 |
Goriaev and Gimandinov (1964) |
|
Artemisia brevifolia |
6.0 |
14.0 |
20.0 |
Goriaev and Gimandinov (1964) |
|
Artemisia campestris |
4.0 |
- |
4.0 |
Guven (1963) |
|
Artemisia capillaris |
- |
- |
- |
Miyazawa and Kameoka (1977) |
|
Artemisia coerulescens |
39.2 |
18.0 |
57.2 |
Sacco, Frattini, and Bicchi (1983) |
|
Artemisia fukudo |
40.0 |
13.0 |
53.0 |
Miyazawa and Kameoka (1977) |
|
Artemisia japonica |
trace |
- |
trace |
Miyazawa and Kameoka (1977) |
|
Artemisia klotzchiana |
- |
33.8 |
33.8 |
Manjarrez and Medina (1964) |
|
Artemisia kurramensis |
- |
55.0 |
55.0 |
Fujita, Ueda, and Maruyama (1963) |
|
Artemisia kurramensis |
- |
62.0 |
62.0 |
Miyazawa and Kameoka (1977) |
|
Artemisia maritima |
31.5 |
15.5 |
47.0 |
Miyazawa and Kameoka (1977) |
|
Artemisia nilagirica |
0.58 |
0.23 |
0.81 |
Uniyal et al (1985) |
|
Artemisia piacea |
trace |
- |
trace |
Miyazawa and Kameoka (1977) |
|
Artemisia vestita |
- |
5.3 |
5.3 |
Vashit and Handa (1964) |
|
Artemisia vulgaris |
1.0 |
- |
1.0 |
Miyazawa and Kameoka (1977) |
|
Juniperus scopulorum |
0.3 |
0.5 |
0.8 |
|
|
|
28.3 |
14.5 |
42.5 |
Brieskorn and Dalferth (1964) |
|
Salvia triloba |
2.3 |
2.8 |
5.1 |
Brieskorn and Dalferth (1964) |
|
Tanacetum vulgare |
19.4 |
58.0 |
77.4 |
Von Rudloff (1964) |
|
Thuja occidentalis |
55.0 |
9.5 |
64.5 |
Von Rudloff (1964) |
|
Thuja orientalis |
5.6* |
- |
5.6 |
Vashist and All. (1963) |
|
Thuja plicta |
70-80 |
5-10 |
75-90 |
Hatch et al (1970) |
|
Tsuga canadensis |
1.3 |
- |
1.3 |
Shaw (1951) |
This chart expanded from Pinto-Scognamiglio (1967).
VAN GOGH AND ABSINTHE
Although the Dutch postimpressionist Vincent Van Gogh is now highly acclaimed, he received little recognition in his lifetime. Instead, he lead a difficult life which included depression, bizarre psychiatric symptoms, and finally suicide. Van Gogh's difficult life --with all its romantic and tragic elements -- has been the focus of much medical speculation. His unusual painting style, psychiatric internment, and voluminous correspondence have proven fertile ground for the theories of modern physicians seeking to diagnosis his ailments. Some of these theories have suggested that some kind of drug-induced intoxication was responsible for his painting style and medical symptoms.
Arnold (1988) has argued that Van Gogh's absinthe drinking played an important role in his illness. In various publications, he (and his colleagues) have suggested that Van Gogh suffered from acute intermittent porphyria (Bonkovsky et al., 1992; Loftus and Arnold, 1991). In this syndrome, a genetic defect in hepatic heme synthesis causes attacks. Symptoms during these attacks can include acute abdominal pain, anxiety, hysteria, delirium, phobias, psychosis, organic disorders, agitation, depression, and altered consciousness from tiredness to coma (Burgovne et al., 1995). Sometimes only one or a few of these symptoms are present during an attack. Attacks can be brought on by nutritional and environmental factors. Van Gogh's fasting, overworking, malnutrition, and alcohol and absinthe use could have all contributed to triggering attacks.
If Van Gogh did indeed have acute intermittent porphyria, absinthe could have played a particularly significant role. In an in vitro study using chick embryo liver cells, Bonkovsky et al. (1992) have demonstrated that alpha-thujone and some related terpenes are porphyrogenic. In other words, assuming terpenes accumulate to a sufficient extent in the liver, drinking absinthe could trigger attacks in someone with a genetic defect in hepatic heme synthesis. This theory seems particularly compelling because it explains Van Gogh's symptoms, the age of onset of his problems, the diseases suffered by other family members, and the role of his lifestyle in contributing to his illness.
Arnold (1988) has further suggested that Van Gogh's fondness for absinthe developed into a generalized craving for thujone and related terpene molecules. Van Gogh is known to have used large amounts of camphor (another terpene) in an attempt to treat insomnia. Furthermore, there are several instances of bizarre behavior which (attempts to eat paint and drink turpentine and kerosene) Arnold sees as evidence for this craving. Although this idea is interesting, there is no strong evidence that terpenes are addictive in this manner. I also wonder if Arnold is not reading too much into Van Gogh's behavior. Not every action of mentally ill or delirious individuals can be explained, after all. Still, even if Van Gogh were only addicted to the alcohol in absinthe, such an addiction could conceivably have kept Van Gogh see-sawing between alcohol withdrawal and porphyric attacks.
Other authors have focused on Van Gogh's paintings and whether his illness or drug use might have contributed to his creativity. One theory (Lee 1981) is that the bright yellow hues and character of his later works were the result of digitalis intoxication, which can produce xanthopsia (yellow vision) and coronas (glowing haloes around objects). Although there is no definite record of Van Gogh receiving this drug, it was used as a treatment for epilepsy and Van Gogh twice painted his physician holding a fox glove (Digitalis purpurea) plant, from which digitalis is extracted. Santonin, which is found in low concentrations in Artemisia absinthium and Artemisia pontica, is also known to cause xanthopsia. However, Arnold and Loftus (1991) have determined that the amount of santonin in absinthe would have been insufficient to produce xanthopsia. Santonin therefore cannot be an explanation for Van Gogh's painting style, while digitalis may have played some role.
In general, I think this type of speculation on the contribution of drugs and illness to art is unproductive. It often seems to rely on an underlying assumption that breaks from 'realistic' representation require some explanation other than the artist's intent. Despite these reservations, I think a case can be made that Van Gogh's experience with mental illness and absinthe intoxication may have influenced his painting. As Arnold and Loftus (1991) write:
...novel experiences of relative sizes, shapes, and colours perceived under the influence of absinthe may have been recalled later and incorporated into new and daring compositions, perspectives, and palettes (p. 507).
Other artists have been known to find inspiration in drug use. However, the connection between creativity and drug intoxication is complex. Drug taking is a social activity. At the same time as an artist is taking a drug, he or she is often interacting with some social group with its own beliefs, behaviors, and aesthetics. When Van Gogh began to drink absinthe, he probably did so in a cafe frequented by writers and artists. It is impossible to separate the artistic influence of that stimulating social environment from whatever effects absinthe could have had on his perception and creativity.
OTHER ARTISTS AND ABSINTHE
Famous absinthe users include:
- Edouard Manet
- Charles Baudelaire
- Paul Verlaine
- Arthur Rimbaud
- Oscar Wilde
- Ernest Dowson
- Edgar Degas
- Henri de Toulouse-Lautrec
- Vincent Van Gogh
- Adolphe Monticelli
- Paul Gauguin
- Alfred Jarry
- Pablo Picasso
- Ernest Hemingway
Visual artworks inspired by absinthe include (expanded from Max's (1990) list.):
- Edouard Manet's 1859 The Absinthe Drinker
- Jean-Francois Raffaelli's 1861 Absinthe Drinkers
- Honore Daumier's 1863 Absinthe Lithographs
- Edgar Degas' 1876 L'Absinthe
- Henri de Toulouse-Lautrec 1887 Portrait of Van Gogh
- Vincent Van Gogh's 1887 Still Life with Absinthe
- Henri de Toulouse-Lautrec 1887 Portrait of Van Gogh (pastel)
- Henri de Toulouse-Lautrec 1893 Monsieur Boileau at the Cafe
- Pablo Picasso 1901 Harlequin and his Companion
- Pablo Picasso 1901 The Poet Cornuty
- Pablo Picasso 1901 The Absinthe Drinker
- Pablo Picasso 1902 The Absinthe Drinker
- Pablo Picasso 1911 Glass of Absinthe
- Pablo Picasso 1914 Absinthe Glass
REFERENCES AND OTHER SOURCES OF INFORMATION:
References Cited in Text:
- Albert-Puleo M. (1978) "Mythobotany, pharmacology, and chemistry of thujone-containing plants and derivatives" Economic Botany 32: 65-74.
- Albert-Puleo M. (1981) "Van Gogh's vision: thujone intoxication [letter]" Jama 246(1):42.
- Arctander (1960) Perfume and Flavor Material of Natural Origin Elizabeth, NJ.
- Arnold, WN (1992) Vincent van Gogh: Chemicals, crises, and creativity Birkhauser; Berlin, Federal Republic of Germany.
- Arnold WN. (1989) "Absinthe" Scientific American 260(6):112-7.
- Arnold WN. (1988) " Vincent van Gogh and the thujone connection Jama 260(20):3042-4.
- Arnold WN; Loftus LS.(1991), "Xanthopsia and van Gogh's yellow palette" Eye 5 ( Pt 5):503-10.
- Bonkovsky HL; Cable EE; Cable JW; Donohue SE; White EC; Greene YJ; Lambrecht RW; Srivastava KK; Arnold WN. (1992) "Porphyrogenic properties of the terpenes camphor, pinene, and thujone (with a note on historic implications for absinthe and the illness of Vincent van Gogh)" Biochemical Pharmacology 43(11):2359-68
- Browne RG; Weissman A. (1981) "Discriminative stimulus properties of delta-9-tetrahydrocannabinol: mechanistic studies" Journal of Clinical Pharmacology 21:227S-234S.
- Burgovne K; Swartz R; Ananth J. (1995) "Porphyria: reexamination of psychiatric implications." Psychotherapy and Psychosomatics, 64(3-4):121-30.
- Caius JF; Mahaskar KS (1920) "The correlation between the chemical composition of antihelmintics and their therapeutic values in connection with the hookworm in the Madras Presidency. II. oleum chenopodii. III oleum absinthium. IV. oleum tanaceti"Indian J. Med. Research 7:570-609.
- Campbell, C. (1987) The romantic ethic and the spirit of modern consumerism Oxford : Basil Blackwell.
- Conrad III, B. (1988) Absinthe : history in a bottle San Francisco : Chronicle Books.
- Corelli, M. (1890?) Wormwood: a drama of paris New York and London : Street & Smith.
- Dehal SS; Croteau R. (1987) "Metabolism of monoterpenes: specificity of the dehydrogenases responsible for the biosynthesis of camphor, 3-thujone, and 3-isothujone" Archives of Biochemistry and Biophysics 258(1):287-91.
- del Castillo, J; Anderson, M; Rubottom, GM. (1975) "Marijuana, absinthe and the central nervous system" Nature 253: 365-366.
- Delahaye, M. (1983) L'absinthe : histoire de la fee verte Paris : Berger-Levrault.
- De Smet PAGM. (19??) "Legislatory outlook on the safety of herbal remedies" in (De Smet PAGM; Keller K; Hansel R; Chandler RF (eds.) Adverse Effects of Herbal Drugs, Vol II, New York : Springer-Verlag.
- Duke, JA (1985) CRC handbook of medicinal herbs Boca Raton, Florida: CRC Press, Inc.
- Erichsen-Brown (1979).
- Galli CL; Galli G; Tragni E; Caruso D; Fiecchi A. (1984) "Quantitative
analysis of
 ,
, 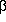 -thujone,
pulegone, safrole, coumarin and
-thujone,
pulegone, safrole, coumarin and 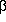 -asarone
in alcoholic beverages by selected-ion monitoring" Journal of Applied
Toxicology 4(5):273-6.
-asarone
in alcoholic beverages by selected-ion monitoring" Journal of Applied
Toxicology 4(5):273-6. - Gilani AH. (1994) "Search for new calcium channel blocking drugs from indigenous plants" International Congress on Natural Products Research August 1-5, Halifax 0:29.
- Gilani AH; Janbaz KH. (1995) "Preventative and curative effects of Artemisia absinthium on acetaminophen and CCl4-induced hepatotoxicity" Gen. Pharmac 26(2):309-315.
- Greenberg JH; Mellors A; McGowan JC. (1978) "Molar volume relationships and the specific inhibition of a synaptosomal enzyme by psychoactive cannabinoids. Journal of Medicinal Chemistry 21(12):1208-12.
- Grieve (1931)
- Guenther, E. (1952) The Essential Oils Vol. 5, Van Nostrand.
- Hach V., Lockhart, R.W., McDonald, E.C., and Cartlidge, D.M. (1970) Can J. Chem. 49, 1762.
- Helig, S. (1894) "Absinthe drinking" Atlanta Constitution, 19 Aug 1894, 6.
- Hemingway, E. (193?) For whom the bell tolls.
- Hernandez, H; Mendiola, J; Torres, D; Garrido, N. (1990) "Effect of aqueous extracts of Artemisia on the in vitro culture of plasmodium falciparum" Fitoterapia 31(6), 540-541.
- Ikram M; Shafi N; Mir I; Do MN; Nguyen; LeQuesne PW. (1987) "24-Ethylcholesta-7,22-Dien-3B-ol: a possibly antipyretic constituent of Artemisia absinthium" Planta Medica ??????.
- Ishida T; Toyota M; Asakawa Y. (1989) "Terpenoid biotransformation in mammals. V. Metabolism of (+)-citronellal, (+-)-7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal, cuminaldehyde, thujone, and (+-)-carvone in rabbits" Xenobiotica, 19(8):843-55.
- Kaul VK; Nigam SS; Dhar KL. (1976) "Antimicrobial activities of the essential oils of Artemisia absinthium linn, Artemisia vestita wall' amd Artemisia vulgaris Linn" Indian Journal of Pharmacy, 38(1):21-22.
- Kissin B. (1974) "Interactions of ethyl alcohol and other drugs" in The Biology of Alcoholism, Vol. 3: Clinical Pathology (B. Kissin and H. Begleiter, eds), Plenum Press, New York.
- Lanier, D. (1995) Absinthe: the cocaine of the nineteenth century Jefferson, NC : McFarland.
- Lee TC. (1981) "Van Gogh's vision. Digitalis intoxication?" Jama, 245(7):727-9.
- Loftus LS; Arnold WN. (1991) "Vincent van Gogh's illness: acute intermittent porphyria? " Bmj 303(6817):1589-91.
- Maruzzella JC; Scrandis DA; Scrandis JB; Grabon G. (1960) Action of odiferous organic chemicals and essential oils on wood-destroying fungi. Plant Disease Reporter 44(10):789-792.
- Max, B. (1990) "This and that" TiPS 11 (Feb), 58-60.
- Meschler J; Marsh C; Land B; Howlett.(1997) "Failure of the active component of absinthe (Artemisia absinthium) to bind the cannabinoid receptor" International Cannabinoid Research Society, 1997 Meeting.
- Miller, R. A. (1993) The Magical and Ritual Use of Herbs, Rochester, VT: Destiny Books.
- Millet Y; Jouglard J; Steinmetz MD; Tognetti P; Joanny P; Arditti J.(1981) "Toxicity of some essential plant oils. Clinical and experimental study" Clinical Toxicology 8(12):1485-98.
- Murphy, R. B. and Schneider, L. H. (1992) Soc. Neurosci. Abstr., Vol. 18, Part 1, p. 180.
- Murphree, HB. (1971) "The importance of congeners in the effects of alcoholic beverages" in Biological Basis ofAlcoholism (Y. Israel and J. Mardones, eds), p. 209-234.
- O'Mallet P; Mugford S. (1991) "The demand for intoxicating commodities: implications for the 'war on drugs'" Social Justic 18(4):122-146.
- Ott, J. (1993) Pharmacotheon Kennewick, WA : Natural Products Co.
- Pal, DC; Jain, SK (1989) "Notes on Lodha medicine in Midnapur District, West Bengal, India" Economic Botany 43(4): 464-470.
- Panayotopoulos, DJ; Chisholm, DD. (1970) "Hallucinogenic effects of nutmeg" British Medical Journal 21 March 1970, 754.
- Pendell, D. (1995) Pharmako/Poeia San Francisco : Mercury House.
- Perez-Souto N; Lynch RJ; Measures G; Hann JT. (1992) "Use of high-performance liquid chromatographic peak deconvolution and peak labelling to identify antiparasitic components in plant extracts" Journal of Chromatography 593:209-215.
- Pinto-Scognamiglio, W. (1967) "Connaissances actuelles sur l'activite pharmacodynamique de la thuyone aromatisant naturel d'un emploi etendu" [Current knowledge on the pharmacodynamic activity of the prolonged administration of thujone, a natural flavoring agent] Bollettino Chimico Farmaceutico 106, 292-300.
- Pinto-Scognamiglio, W. (1968) "Effetti del tujone sull'attivita' spontanea e sul comportamento condizionato del ratto" [Effects of thujone on spontaneous activity and on conditioned behavior of rats]Bollettino Chimico Farmaceutico 107, 780-791.
- Prestwich, P. (1988) Drink and the politics of social reform: antialcoholism in france since 1870 Palo Alto, CA : Society for the Promotion of Science and Scholarship.
- Pyes, C. (197?) "Absinthe" High Times, p. 62-66,72.
- Ratsch, C. (1995) "Absinth in der schweiz"Yearbook for Ethnomedicine and the Study of Consciousness 4:285-288
- Rice KC; Wilson RS. (1976) "(-)-3-Isothujone, a small nonnitrogenous molecule with antinociceptive activity in mice"Journal of Medicinal Chemistry 19(8):1054-7.
- Shulgin AT. (1966) Nature 210:380.
- Simonetti, Gualtiero (1990) Simon and Schuster's Guide to Herbs and Spices, Simon and Schuster.
- Simonsen, J. L. (1949) The Terpenes Vol. 2, Univ. Press.
- Steinriede, K. (1996) "Lickerish liqueur: anise to absinthe to anisette" au Juice 3:64-67.
- Vogt, D. D. and Montagne, M. (1982) "Absinthe: behind the emerald mask" Int. J. Addict 17, 1015-1029.
- Zafar MM; Hamdard; Hameed A. (1990) "Screening of Artemisia absinthium for antimalarial effects on plasmodium berghei in mice: a preliminary report" Journal of Ethnopharmacology 30:223-226.

