Impaired cognitive performance in drug free users of recreational ecstasy (MDMA)
Euphrosyne Gouzoulis-Mayfranka, Jörg Daumanna, Frank Tuchtenhagena, Susanne Pelza, Steffanie Beckera, Hans-Jürgen Kunerta, Bruno Fimmb, Henning Sassa
a Department of
Psychiatry and Psychotherapy, Medical Faculty of the University of
Technology (RWTH), Pauwelsstrasse 30, D-52074 Aachen, Germany, b Department of Neurology
Correspondence to: Dr E Gouzoulis-Mayfrank, Department of Psychiatry and Psychotherapy, Medical Faculty of the University of Technology (RWTH), Pauwelsstrasse 30, D-52074 Aachen, Germany email egouzoulis@post.klinikum.rwth-aachen.de
Received 8 July 1999 and in revised form 4 November 1999;
Accepted 11 November
1999
| |
Abstract |
|---|
|
|
|---|
OBJECTIVES Ecstasy
(3,4-methylenedioxymethamphetamine (MDMA) and related congerers:
MDA, MDEA) is the name given to a group of popular recreational drugs.
Animal data raise concern about neurotoxic effects of high doses of
ecstasy on central serotonergic systems. The threshold dose for
neurotoxicity in humans is not clear and serotonin is involved in
several functions including cognition. The purpose of this study was to
investigate cognitive performance in a group of typical recreational
ecstasy users.
Ecstasy
(3,4-methylenedioxymethamphetamine (MDMA) and related congerers:
MDA, MDEA) is the name given to a group of popular recreational drugs.
Animal data raise concern about neurotoxic effects of high doses of
ecstasy on central serotonergic systems. The threshold dose for
neurotoxicity in humans is not clear and serotonin is involved in
several functions including cognition. The purpose of this study was to
investigate cognitive performance in a group of typical recreational
ecstasy users.
METHODS A
comprehensive cognitive test battery was administered to 28 abstinent
ecstasy users with concomitant use of cannabis only and to two equally
sized matched groups of cannabis users and non-users. The sample
consisted of ecstasy users with a typical recreational use pattern and
did not include very heavy users.
A
comprehensive cognitive test battery was administered to 28 abstinent
ecstasy users with concomitant use of cannabis only and to two equally
sized matched groups of cannabis users and non-users. The sample
consisted of ecstasy users with a typical recreational use pattern and
did not include very heavy users.
RESULTS Ecstasy users
were unimpaired in simple tests of attention (alertness). However, they
performed worse than one or both control groups in the more complex
tests of attention, in memory and learning tasks, and in tasks
reflecting aspects of general intelligence. Heavier ecstasy and heavier
cannabis use were associated with poorer performance in the group of
ecstasy users. By contrast, the cannabis users did not differ
significantly in their performance from the non-users.
Ecstasy users
were unimpaired in simple tests of attention (alertness). However, they
performed worse than one or both control groups in the more complex
tests of attention, in memory and learning tasks, and in tasks
reflecting aspects of general intelligence. Heavier ecstasy and heavier
cannabis use were associated with poorer performance in the group of
ecstasy users. By contrast, the cannabis users did not differ
significantly in their performance from the non-users.
CONCLUSIONS The
present data raise concern that use of ecstasy possibly in conjunction
with cannabis may lead to cognitive decline in otherwise healthy young
people. Although the nature of the emerging cognitive disturbance is
not yet clear, an impairment of working memory might be the common
denominator underlying or contributing to declines of performance in
various tasks. The cognitive disturbance is likely to be related to the
well recognised neurotoxic potential of ecstasy. The data suggest that
even typical recreational doses of ecstasy are sufficient to cause
neurotoxicity in humans.
The
present data raise concern that use of ecstasy possibly in conjunction
with cannabis may lead to cognitive decline in otherwise healthy young
people. Although the nature of the emerging cognitive disturbance is
not yet clear, an impairment of working memory might be the common
denominator underlying or contributing to declines of performance in
various tasks. The cognitive disturbance is likely to be related to the
well recognised neurotoxic potential of ecstasy. The data suggest that
even typical recreational doses of ecstasy are sufficient to cause
neurotoxicity in humans.
(J Neurol Neurosurg Psychiatry 2000;68:719-725)
Keywords:
ecstasy;
3,4-methylenedioxymethamphetamine;
neurotoxicity;
cognitive
performance
| |
Introduction |
|---|
|
|
|---|
Ecstasy (3,4-methylenedioxymethamphetamine (MDMA) and related congerers MDA, MDEA) is the name given to a group of popular recreational drugs with neurotoxic effects on central serotonergic systems. Reductions of serotonin (5-HT), its metabolite 5-HIAA, and 5-HT transporter binding in brain tissue as well as diminished activity of tryptophan hydroxylase have been demonstrated in several species including non-human primates after administration of relatively high and repeated doses of MDA and MDMA.1-5 Neuroanatomical studies disclosed widespread degeneration of serotonergic axon terminals throughout the whole brain.6-8 Although recovery from neurotoxic damage was almost complete in most rats after 12 months,4 in non-human primates neurotoxic brain alterations were still detectable as long as 7 years after MDMA exposure and included hyporegeneration in preminantly cortical and hyperregeneration with aberrant axon sprouting in predominantly subcortical regions.5 9-11 The lowest MDMA dose, which elicited long term structural damage in serotonergic neurons of non-human primates was 5 mg/kg subcutaneously twice daily for 4 days.5 Although some heavy users take ecstasy in quantities that approach those experimental doses, most recreational users do not. The typical recreational user takes one or two ecstasy pills containing about 100-140 mg MDMA each (or equivalent dose of MDA or MDEA) during the weekend and abstains from use during the week. However, the threshold dose for human neurotoxicity is not clear and humans may be more susceptible than primates. In addition, it is possible that the cumulative doses ingested by moderate recreational users over a longer period of regular use bear a similar neurotoxic risk as high experimental doses administered within a short period of time.
In a recent PET study with the selective 5-HT transporter ligand McN-5652 abstinent ecstasy users showed decreased brain 5-HT transporter binding compared with controls and this decrease correlated with the extent of previous ecstasy use.12 This was the first in vivo demonstration of long term and, therefore, probably neurotoxic brain damage in ecstasy users. However, the possible functional consequences of this toxic brain injury have not yet been elucidated. Serotonergic systems are involved in numerous functions including regulation of mood and drive, cognition, vegetative function, pain, and neuroendocrine secretion. Although the role of serotonin in cognition is not clear, some studies indicate that diminished serotonergic activity may cause cognitive impulsivity with higher rates of anticipatory responses in reaction time tasks and may interfere with memory processes.13-18 Some recent studies considered the question of cognitive performance in drug free ecstasy users. One study demonstrated an impulsive cognitive style with high error rates,19 whereas most studies demonstrated moderate, subclinical memory problems in ecstasy users.20-25 More recently, subtle deficits in tasks requiring sustained and complex attention were also reported.26 However, methodological problems of previous studies included comorbidity with other psychiatric disorders besides drug use,20 lack of data on the use of other illicit drugs21 or polydrug use,20 23 and short abstinence periods of only 4-7 days before the study day, thus raising the question of pharmacological rather than long term toxic effects.21-23 In a recent study of memory performance decrements were demonstrated only in heavy ecstasy users who had a concomitant use of various other illicit drugs (polydrug users), but not in moderate ecstasy users.24 The aim of the present study was to assess various cognitive abilities in abstinent recreational ecstasy users who did not previously use ecstasy in extremely high doses and who were not polydrug users.
| |
Methods |
|---|
|
|
|---|
SUBJECTS
We enrolled 28 ecstasy users who reported its regular use over 6 months or longer with a minimum frequency of twice a month within the
past 2 years or use of ecstasy on at least 25 occasions during the past
2 years (inclusion criteria). Recruitment was performed directly in the
dance scene by students who were involved in the study and via word of
mouth. Exclusion criteria were: (1) regular use of other legal or
illegal psychotropic drugs with the exception of cannabis (regular use
was defined as use once a month or more often over 6 months or longer
within the past 2 years), and (2) regular heavy use of alcohol (defined
as severe drunkenness occurring at a frequency of at least twice a
month). Because almost every ecstasy user smokes cannabis, it was
impossible to recruit a reasonable number of "exclusive" ecstasy
users. Therefore, we enrolled two control groups. The first one
consisted of 28 healthy persons who had never taken ecstasy and had no
previous or current history of regular drug use or regular heavy
alcohol use (definitions according to the exclusion criteria of the
ecstasy user group). The second control group consisted of 28 persons who had never taken ecstasy and were matched for cannabis use with the
ecstasy user group, but had no previous or current history of regular
use of other drugs or regular heavy alcohol use (definitions also
according to the exclusion criteria of the ecstasy user group). This
second control group is termed the "group of cannabis users", although five subjects had only sporadic or no use of cannabis at all
(because six ecstasy users had also only sporadic or no use of
cannabis). Both control groups were matched for age, sex, and education
with the ecstasy user group. Exclusion criteria for all participants
were any current or previous axis I psychiatric diagnoses (except for
drug misuse in the two user groups), any organic brain disorder, any
relevant general medical disease requiring pharmacological treatment,
and any medication on the study day. Subjects were screened for
inclusion and exclusion criteria by means of a preliminary telephone
interview, which was followed by a personal interview and medical
history including the structured interview for the diagnostic and
statistical manual of mental disorders IV (DSM-IV) and a detailed
history of drug misuse. The ecstasy users agreed to abstain from
ecstasy use for at least 7 days before the study. Ecstasy and cannabis
users agreed to abstain from use of cannabis on the study day. Drug
screens were performed on the study day with urine samples for the
following substance groups: amfetamines, methamfetamines, cocaine and
its metabolite, marijuana, benzodiazepines, barbiturates, and opiates. A positive screen for any substance except for cannabis was an exclusion criterion. After complete description of the study to the
subjects, written informed consent was obtained and subjects were paid
for their participation. The study was approved by the local ethics
committee at the RWTH Aachen.
NEUROPSYCHOLOGICAL TEST BATTERY
All subjects underwent a session composed of both computerised and
paper and pencil tests. The session began at about 900 am, lasted about
3 hours, and was interrupted by a pause of 15 minutes half way. The
test battery consisted of the following tests:
TESTS OF ATTENTION
Tonic and phasic alertness (test for attentional performance
TAP,27 subtest 1)
A simple reaction time (RT) task measuring response readiness to a
simple visual target which appears on the computer screen preceded or
not preceded by a warning acoustic signal.
Selective visual attention (TAP, subtest 6)
A more complex RT task requiring visual memory, target selection,
and response inhibition (go/nogo). Five relatively similar complex
figures are presented on the computer screen and two out of those five
figures are defined as the critical targets. Subjects have to memorise
the figures and thereafter they have to react to the critical targets
by pressing a computer key and ignore the non-critical targets.
Divided attention (TAP, subtest 5)
A demanding dual RT task requiring attention to simultaneously
presented visual and acoustic cues. Subjects have to respond to the
appearance of a square composed of small crosses among other irregular
shapes on the screen and to any irregularity occurring in an alternate
sequence of high and low tones.
Intermodal integration (TAP, subtest 8)
Subjects view upward or downward directed arrows on the screen and
simultaneously listen to high and low tones. They have to react by
pressing a computer key whenever they detect a match (for example,
simultaneous appearance of an upward directed arrow and a high tone).
Visual scanning (TAP, subtest 12)
Subjects scan a matrix of 5×5 similar graphic elements for a
target, which is presented before the onset of the trial.
Cognitive interference
The classic Stroop test was administered in the German
standardised paper and pencil version.28
TESTS OF MEMORY SPAN AND WORKING MEMORY
Corsi block tapping test
This classic test29 assesses the visuospatial memory
span. Subjects memorise and reproduce a series of spatial locations by
touching prearranged wooden blocks in the sequence previously demonstrated by the examiner.
Digit span (Wechsler adult intelligence scale-revised
WAIS-R, German version,30 subtest 3)
Subjects repeat a list of orally presented digits forward (verbal
memory span) or backward (verbal working memory).
TESTS OF MEMORY AND LEARNING
VLMT: verbal learning and memory test31
This is a German standardised equivalent of the classic auditory
verbal learning test.32 A learning list of 15 words is presented orally by the experimenter. Assessments include free recall
after the first presentation (immediate memory span), learning performance with a maximum five consecutive presentations, decrement of
performance after presentation of an interference word list of 15 words, and recognition performance by means of a third longer word list
containing the words of the learning list and a larger number of other
words not presented previously. Recognition performance is assessed 30 minutes after the last presentation of the learning list.
VIG: visuospatial memory33
This computerised visuospatial equivalent of the VLMT assesses
immediate memory span and learning performance for complex visual
arrangements consisting of geometric figures.
PREFRONTAL AND GENERAL INTELLIGENCE TESTS
Word fluency
In this variation of the classic frontal Benton word fluency
task subjects generate as many words of specific phonological and
semantic criteria as possible within a minute (initial letter, semantic
category, alternating criteria: phonological/ phonological, semantic/semantic, phonological/semantic).
LPS-4: abstract logical thinking (from the computerised version
of the Leistungsprüfsystem LPS)33 34
In this problem solving task subjects have to find out the rule in
a series of digits and letters and to indicate the "wrong" element
which is violating the rule (fluid intelligence).
Mosaic test (WAIS-R, German version,30 subtest 6)
This is another classic test of fluid intelligence assessing
visuomotor performance, planning, and problem solving. Subjects must
reproduce complex visual patterns with cubes.
General knowledge (WAIS-R, German version,30 subtest
1)
Subjects answer 24 questions of general knowledge. Once acquired,
general knowledge remains relatively unaffected for a long period of
time after the onset of any organic cognitive decline. Therefore, it is
thought to reflect the crystallised by contrast with the more
susceptible fluid portion of intelligence.35
STATISTICS
Reaction times (RT: medians and variance), errors, anticipations
(RT<100s), and performance scores of the three groups were analysed by
means of analysis of variance (ANOVA) and Scheffé post-hoc tests. In
addition, a canonical discriminant analysis was performed with the
entire data set of all subjects (reaction times, error rates, and
performance scores of the complete test battery: 48 values in total).
The relation between the performance data of the ecstasy users and
their previous ecstasy and cannabis use was analysed with Pearson's
correlation coefficient. Because of the possible effects of
pre-existing differences in general intelligence and educational level
the ANOVA was rerun using the general knowledge score as a covariate
(ANCOVA). In addition, the relation between the three intelligence
scores and the significant scores in the attentional and memory tasks
were analysed with the Pearson's correlation coefficient in the
ecstasy user group. p Values<0.05 were considered significant. All
procedures were performed using SPSS version 7.5.
| |
Results |
|---|
|
|
|---|
The three groups were similar in terms of sex distribution, age,
and educational level (table 1). Although the level of education was
slightly lower in the ecstasy group, the differences were not
statistically significant (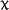 2=6.44, df=8, p=0.59,
educational levels 1-3 and 4-5 were pooled together for testing). The
data for ecstasy and cannabis use are given in table 2. The two user
groups were similar for the extent of previous use of cannabis. Ecstasy
users had used ecstasy over 27 months on average. The average estimated
total dose was 93.4 ecstasy tablets. Hence the average extent of use
was about 3.5 ecstasy tablets a month, which is a typical recreational
and not a very heavy use. Most ecstasy users had taken the drug for the last time 2 to 8 weeks before the study. In four cases this time period
was shorter (7-10 days) whereas in two cases it was considerably longer (6-12 months).
2=6.44, df=8, p=0.59,
educational levels 1-3 and 4-5 were pooled together for testing). The
data for ecstasy and cannabis use are given in table 2. The two user
groups were similar for the extent of previous use of cannabis. Ecstasy
users had used ecstasy over 27 months on average. The average estimated
total dose was 93.4 ecstasy tablets. Hence the average extent of use
was about 3.5 ecstasy tablets a month, which is a typical recreational
and not a very heavy use. Most ecstasy users had taken the drug for the last time 2 to 8 weeks before the study. In four cases this time period
was shorter (7-10 days) whereas in two cases it was considerably longer (6-12 months).
|
|
In the reaction time (RT) tasks there were only very few errors and
anticipatory responses in all three groups and there were no
statistical differences among the groups. Therefore, these data are not
presented. The overall results (RT and performance data) showed mostly
good cognitive levels in all three groups. However, the ecstasy users
tended to perform poorer in most tests and there were some
statistically significant differences between the groups (ANOVA, table
3): in the test of selective visual attention (go/nogo) ecstasy users
had longer RTs than both control groups and a larger variance of RTs
than the non-user group. In the tests of divided attention and
intermodal integration ecstasy users had significantly longer RTs than
the cannabis users. In the verbal working memory task (digit span
backward) ecstasy users performed poorer than non-users. In the verbal
learning and memory test (VLMT) ecstasy users recalled less words after
the first presentation of the learning list than non-users (immediate
recall). They required more repetitions to learn the items than both
control groups and they forgot more words after the presentation of the interference list than the non-users. In the visuospatial memory task
(VIG) ecstasy users recalled less figures after the first presentation
than both control groups (immediate recall). Finally, ecstasy users
showed a poorer performance than both control groups in all three
general intelligence tests. Scores on the questionnaire for the
assessment of concentration and memory problems did not differ
significantly between the three groups, although the two user groups
tended to score higher than the non-user group. By means of the
canonical discriminant analysis 90.36% of the subjects could be
assigned correctly to one of the three groups according to their
performance data (92.9% of the ecstasy users, 85.7% of the cannabis
users, and 92.6% of the non-users).
|
In the group of ecstasy users long RTs in the divided attention task
were associated with a long period of regular ecstasy use
(r=0.411, p<0.05). A poor digit span
performance (working memory) was associated with a higher estimated
cumulative ecstasy dose (r= 0.382,
p<0.05) and a young age of onset of cannabis use (r=0.518, p=0.01). Poor performance in the
VLMT (verbal memory and learning) was associated with heavier ecstasy
use (immediate recall/estimated cumulative dose:
r=
0.382,
p<0.05) and a young age of onset of cannabis use (r=0.518, p=0.01). Poor performance in the
VLMT (verbal memory and learning) was associated with heavier ecstasy
use (immediate recall/estimated cumulative dose:
r= 0.387, p<0.05; interference effect by
second word list/frequency of use: r=0.425,
p<0.05; number of repetitions in the learning phase/average one night dose: r=0.489, p<0.01) and with heavier
cannabis use (number of repetitions/frequency of use:
r=0.577, p<0.01). No other correlation of
any test score with any aspect of the pattern of ecstasy or cannabis
use reached statistical significance.
0.387, p<0.05; interference effect by
second word list/frequency of use: r=0.425,
p<0.05; number of repetitions in the learning phase/average one night dose: r=0.489, p<0.01) and with heavier
cannabis use (number of repetitions/frequency of use:
r=0.577, p<0.01). No other correlation of
any test score with any aspect of the pattern of ecstasy or cannabis
use reached statistical significance.
In the analysis of covariance (ANCOVA) using the general knowledge score as the covariate group differences were still significant for all previously significant performance scores except for the interference effect by the second word list in the verbal memory task (VLMT). In this particular case the group effect only approached significance (p=0.092) in the covariance analysis. In addition, there was a significant, although not very high correlation between the three intelligence test scores (general knowledge/mosaic test: r=0.386, p=0.043; mosaic test/LPS-4: r=0.390, p=0.040), but no correlation between any of the intelligence tests and the other performance scores in the ecstasy user group (n=28).
| |
Discussion |
|---|
|
|
|---|
A comprehensive cognitive test battery was administered to 28 recreational ecstasy users with concomitant use of cannabis only and to two equally sized matched groups of cannabis users and non-users. The test battery included tests of attention, memory and learning, frontal lobe function, and general intelligence. Although all three groups performed well within the normal range ecstasy users performed worse than one or both control groups in the more complex tests of attention (go/nogo task of selective attention, divided attention, and intermodal integration), in memory and learning tasks, and in the tasks reflecting aspects of general intelligence. By contrast, performance in simple reaction time tasks of attention (tonic and phasic alertness) was unaffected. Poorer performance scores or longer reaction times in the working memory, verbal memory, and divided attention tasks were associated with heavier ecstasy and heavier cannabis use. These results raise the concern that ecstasy use even in typical moderate recreational doses and possibly in conjunction with cannabis use may lead to a subclinical cognitive decline in otherwise healthy young people. Although we cannot rule out the possibility that concomitant regular use of other legal and illegal drugs and/or heavier ecstasy use was concealed by subjects, we do not think that this was the case because they were not aware of our inclusion and exclusion criteria when first interviewed and there was no motivation for them to falsify their data once they agreed to participate in the study. In addition, our clinical impression of the ecstasy users did not fit the typical characteristics of heavy polydrug users.
The ecstasy users reported an average time period of more than 3 weeks from the last intake of ecstasy until the study day. Therefore, purely pharmacological effects of ecstasy or the commonly reported "after effects" in the days after ecstasy ingestion21-23 are unlikely to have determined the poorer performance of ecstasy users compared with the control groups. By contrast, concomitant cannabis use may well have influenced the cognitive performance of ecstasy users. Noteworthy, the two user groups were similar for the extent of previous cannabis use, the only exception being the duration of regular use, which was longer in the group of ecstasy users. However, there was no association between performance data and duration of regular cannabis use, although there were associations between poorer performance and other aspects of the pattern of cannabis use (younger onset age and higher frequency of use). Finally, the time elapsed since the last use of cannabis tended to be somewhat shorter in the cannabis user group. In conclusion, concomitant cannabis use is unlikely to fully account for the poorer performance of ecstasy users compared with the cannabis user group, although cannabis use is likely to have affected cognition and to have contributed to some extent to the poorer performance of ecstasy users compared with the non-user group. However, the data of the cannabis user group demonstrate that cannabis use alone was not sufficient to impair the performance of subjects to a significant degree.
Finally, it might be hypothesised that the poorer performance of ecstasy users was due to accidental pre-existing differences in general cognitive capacity or intelligence. Ecstasy users performed worse not only in the two tests of fluid intelligence (mosaic test, LPS-4: logical thinking, and problem solving) which are likely to be affected by subtle or the start of more serious cognitive deterioration, but also in the general knowledge test, a classic test of crystallised intelligence, which is thought to be insensitive to early cognitive decline.35 Although there was no statistically significant difference in the level of education between the three groups, cannabis users and non-users tended to have slightly higher levels of education on average (highest school leaving examination qualifying for admission to university or university degree, see table 1). However, we do not think that possible pre-existing differences in intelligence and education are sufficient to explain the poorer performance of the ecstasy user group in tests of attention and memory. Firstly, there was a significant correlation between memory and attention scores and the extent of ecstasy use indicating an association of poorer performance with heavier use. Secondly, group effects remained significant when we reran the ANOVA using the score of the general knowledge test as a covariate. Thirdly, there was no correlation between the three intelligence test scores and the performance or reaction time data of the attention and memory tasks. The poorer general knowledge in the ecstasy user group may well be the consequence of the early socialisation within the dance and drug scene, which is likely to have somewhat narrowed the fields of interests and to have interfered with the acquisition of general knowledge. By contrast, the poorer performance of ecstasy users in the two tests of fluid intelligence may reflect either a start of, but still subclinical cognitive decline, or the slightly inferior level of education, or a combination of both factors.
In summary, our data suggest that ecstasy use over a period of months or a few years may cause long term impairment of cognitive performance even when ecstasy is taken in typical recreational and not necessarily very high doses. Concomitant cannabis use may contribute to the impairment. However, the nature of the emerging cognitive disturbance is not yet clear. Besides the possibility of multiple distinct impairments of attention, memory processes, and other cognitive skills it might be hypothesised that one common disturbance underlies or contributes to impairments in various tasks. Increased levels of cognitive and behavioural impulsivity have been associated with conditions with reduced serotonergic function17 37-40 and have been shown in some19 41 but not in all studies42 with ecstasy users. An impulsive cognitive style with poor planning may well impair the performance in complex tasks of attention, as well as in learning tasks and tasks of fluid intelligence. However, our overall data are not supportive of an increased cognitive impulsivity in our sample, because ecstasy users did not demonstrate increased rates of errors and premature reactions (anticipation: RT<100 ms) and had longer rather than shorter reaction times compared with the control groups. The only hints towards this direction were the results in the visual scanning task. Ecstasy users exhibited a shorter processing time than the control groups in the non-critical trials of this task, whereas in the critical trials their processing time correlated poorly with the target position. However, these group differences did not reach significance. Alternatively, the underlying common cognitive disturbance might be a problem of working memory, which refers to the ability of holding information "on line" for short periods of time and manipulating it in the service of guiding behaviour.43 Working memory is a complex skill which cannot be entirely differentiated from processes of attention, memory span, and even general intelligence.35 In our test battery working memory is best reflected by the digit span performance (backward), but, in addition, it is involved in the memory and learning tasks, in both tests of fluid intelligence and in the task of selective attention with response selection (go nogo). Therefore, working memory may be a good candidate for the substrate of cognitive impairment in ecstasy users.
The cognitive disturbance in our sample of ecstasy users is likely to be related to the well recognised neurotoxic potential of ecstasy, which is restricted to the serotonergic system. Although no clear picture has emerged so far about the role of serotonin in cognition, it is involved in various cognitive tasks involving memory and speed of information processing.16 17 44 Moreover, cognitive deterioration in users may be related to secondary regulatory mechanisms involving other neurotransmitter systems that are not directly affected by the neurotoxic potential of the drug. Subclinical cognitive decline may not be noticed by the subjects themselves over a long period of time. Therefore, subjects are likely to continue using ecstasy and put themselves at substantial risk for further deterioration over the years. Theoretically, it is possible that the cognitive impairment becomes apparent only after many years when the effects of normal aging add to the possible neurotoxic damage. A most important question refers to the reversibility or permanence of the adverse cognitive effects after longer periods of abstinence. A recent primate study showing abnormal cerebral 5-HT innervation patterns 7 years after MDMA exposure11 raises concern about the possible irreversibility of functional consequences of serotonergic damage in humans. This question cannot be answered in cross sectional studies and must be considered in future longitudinal investigations. Given the popularity of ecstasy among young people the present data are clearly alarming and underline the need for further research in this field.
| |
Acknowledgments |
|---|
This work was supported by grants to EG-M from the START program at the University of Technology Aachen (RWTH) and from the Deutsche Forschungsgemeinschaft (DFG Go 717/2-1). We thank Professor M J Bogusz, Department of Forensic Medicine of the RWTH Aachen, for the performance of the drug screens.
| |
References |
|---|
|
|
|---|
| 1. |
Ricaurte GA,
Bryan G,
Strauss L,
et al. Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals.
Science
1985;22:986-988 |
| 2. |
Battaglia G,
Yeh SY,
O'Hearn E,
et al. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H] paroxetine-labelled serotonin uptake sites.
J Pharmacol Exp Ther
1987;242:911-916 |
| 3. |
Schmidt CJ. Acute administration of methylenedioxymethamphetamine: comparison with the neurochemical effects of its N-desmethyl and N-ethyl analogs.
Eur J Pharmacol
1987;136:81-88 |
| 4. |
Battaglia G,
Yeh SY,
De Souza EB. MDMA-induced neurotoxicity: parameters of degeneration and recovery of brain serotonin neurons.
Pharmacol Biochem Behav
1988;29:269-274 |
| 5. |
Ricaurte GA,
Martello AL,
Katz JL,
et al. Lasting effects of MDMA on central serotonergic neurons in nonhuman primates: neurochemical observations.
J Pharmacol Exp Ther
1992;261:616-622 |
| 6. |
O'Hearn EG,
Battaglia G,
De Souza EB,
et al. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity.
J Neurosci
1988;8:2788-2803 |
| 7. |
Wilson MA,
Ricaurte GA,
Molliver ME. Distinct morphologic classes of serotonergic axons in primates exhibit differential vulnerability to the psychotropic drug 3,4-methylene amphetamine.
Neuroscience
1989;28:121-137 |
| 8. |
Molliver ME,
Berger UV,
Mamounas LA,
et al. Neurotoxicity of MDMA and related compounds.
Ann NY Acad Sci
1990;600:640-646 |
| 9. |
Ricaurte GA,
Forno LS,
Wilson MA,
et al. MDMA selectively damages central serotonergic neurons in the primate.
JAMA
1988;260:51-55 |
| 10. |
Fischer C,
Hatzidimitriou G,
Wlos J,
et al. Reorganization of ascending 5-HAT axon projections in animals previously exposed to the recreational drug 3,4-methylenedioxyamphetamine (MDMA, ecstasy).
J Neurosci
1995;15:5476-5485 |
| 11. |
Hatzidimitriou G,
McCann DU,
Ricaurte G. Altered serotonin innervation patterns in the forebrain of monkeys treated with +/-3,4-methylenedioxymethamphetamine seven years previously: factors influencing abnormal recovery.
J Neurosci
1999;19:5096-5107 |
| 12. |
McCann DU,
Szabo Z,
Scheffel U,
et al. Positron emission tomographic evidence of toxic effect of MDMA (ecstasy) on brain serotonin neurons in human beings.
Lancet
1998;352:1433-1437 |
| 13. |
Soubrié P. Reconciling the role of the central serotonin neurons in human and animal behavior.
Behav Brain Sci
1986;9:319-364 |
| 14. |
Altman HJ,
Normile HJ. What is the nature of the role of the serotonergic nervous system in learning and memory: prospects for development of an effective treatment strategy for senile dementia.
Neurobiol Aging
1988;9:627-638 |
| 15. |
McEntee W,
Crook T. Serotonin, memory and aging.
Psychopharmacology
1991;103:143-149 |
| 16. |
Sirvio J,
Riekkinen P,
Jakala P Jr,
et al. Experimental studies on the role of serotonin in cognition.
Prog Neurobiol
1994;43:363-379 |
| 17. | Robbins TW, Everitt BJ. Arousal systems and attention. In: Gazzaniga MS, ed. The cognitive neurosciences. London: MIT Press, 2nd printing, 1995:703-20. |
| 18. |
Poulos CX,
Parker JL,
Le AD. Desfenfluramine and 8-OH-DPAT modulate impulsivity in a delay-of-reward paradigm: implications for a correspondence with alcohol consumption.
Behav Pharmacol
1996;7:395-399 |
| 19. |
Morgan MJ. Recreational use of ecstasy (MDMA) is associated with elevated impulsivity.
Neuropsychopharmacology
1998;19:252-264 |
| 20. |
Krystal JH,
Price LH. Chronic 3,4-methylenedioxymethamphetamine (MDMA) use: effects on mood and neuropsychological function?
Am J Drug Alcohol Abuse
1992;18:331-341 |
| 21. |
Curran HV,
Travill RA. Mood and cognitive effects of (3,4-methylenedioxymethamphetamine (MDMA, ecstasy): week-end high followed by mid-week low.
Addiction
1997;92:821-831 |
| 22. |
Parrott AC,
Lees A,
Garnham NJ,
et al. Cognitive performance in recreational users of MDMA (ecstasy): evidence for memory deficits.
J Psychopharmacol
1998;12:79-83 |
| 23. |
Parrott AC,
Lasky J. Ecstasy (MDMA) effects upon mood and cognition: before, during and after a Saturday night dance.
Psychopharmacology
1998;139:261-268 |
| 24. |
Bolla KI,
McCann DU,
Ricaurte GA. Memory impairment in abstinent MDMA (ecstasy) users.
Neurology
1998;51:1532-1537 |
| 25. |
Morgan MJ. Memory deficits associated with recreational use of ecstasy (MDMA).
Psychopharmacology
1999;141:30-36 |
| 26. |
McCann DU,
Mertl M,
Eligulashvili V,
et al. Cognitive performance in +/-3,4-methylenedioxymethamphetamine (MDMA, ecstasy) users: a controlled study.
Psychopharmacology
1999;143:417-425 |
| 27. | Zimmermann P, Fimm B. Test for attentional performance (TAP). Herzogenrath: PsyTest, 1995. |
| 28. | Bäumler G. Farbe-Wort-Interferenztest FWIT. Göttingen: Hogreve, 1985. |
| 29. | Corsi AT. Human memory and the medial temporal region of the brain [dissertation]. Boston: McGill University, 1972. |
| 30. | Tewes U. HAWIE-R. Hamburg-Wechsler Intelligenztest für Erwachsene / Revision 1991., 2nd ed. Bern: Huber, 1991. |
| 31. |
Helmstaedter C,
Durwen HF. VLMT: Verbaler Lern und Merkfähigkeitstest. Ein praktikables und differenziertes Instrumentarium zur Prüfung der verbalen Gedächtnisstungen.
Schweiz Arch Neurol Psychiatr
1990;141:21-30 und Merkfähigkeitstest. Ein praktikables und differenziertes Instrumentarium zur Prüfung der verbalen Gedächtnisstungen.
Schweiz Arch Neurol Psychiatr
1990;141:21-30 |
| 32. | Rey A. L'examen de clinique en psychologie. Paris: Presses Universitaires de France, 1964. |
| 33. | Hänsgen K-D, Merten Th. LEILA: Leistungsdiagnostisches Labor., 3rd ed. Göttingen: Hogreve, 1995. |
| 34. | Horn W. Leistungsprüfsystem L-P-S., 2nd ed. Göttingen: Hogreve, 1983. |
| 35. | Duncan J. Attention, intelligence and the frontal lobes. In: Gazzaniga MS, ed. The cognitive neurosciences. 2nd printing. London: MIT Press, 1995;721-733. |
| 36. | Cramon DY, Mai N, Ziegler W. Neuropsychologische Diagnostik. Weinheim: VCH Verlags gesellschaft, 1993. |
| 37. |
Linnoila M,
Virkkunen M,
Scheinin M,
et al. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from non-impulsive violent behavior.
Life Sci
1983;33:2609-2614 |
| 38. |
Coccaro EF,
Siever LJ,
Klar HM,
et al. Serotonergic studies in patients with affective and personality disorders.
Arch Gen Psychiatry
1989;46:587-599 |
| 39. |
0'Keane V,
Moloney E,
O'Neilly,
et al. Blunted prolactin responses to d-fenfluramine in sociopathy. Evidence for subsensitivity of a central serotonergic function.
Br J Psychiatry
1992;160:643-646 |
| 40. |
Virkkunen M,
Linnoila M. Brain serotonin, type II alcoholism and impulsive violence.
J Stud Alcohol
1993;11(suppl):163-169 |
| 41. |
Gerra G,
Zaimovic A,
Giucastro G,
et al. Serotonergic function after 3,4-methylenedioxymethamphetamine (ecstasy) in humans.
Intern Clin Psychopharmacol
1998;13:1-9 |
| 42. |
Mc Cann DU,
Ridenour A,
Shaham Y,
et al. Serotonin neurotoxicity after 3,4-methymethamphetamine (MDMA, ecstasy): a controlled study in humans.
Neuropsychopharmacology
1994;10:129-138 |
| 43. |
Baddeley A. Working memory.
Science
1992;255:556-559 |
| 44. |
Hasbroucq T,
Rihet P,
Blin O,
et al. Serotonin and human information processing: fluvoxamine can improve reaction time performance.
Neurosci Lett
1997;229:204-208 |
© 2000 by Journal of Neurology, Neurosurgery, and Psychiatry
This article has been cited by other articles:
- KELLY, P. A T
(2000). Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J. Neurol. Neurosurg. Psychiatry
68: 690-690
[Full Text]
Related articles in JNNP:
- Impaired cognitive performance in drug free users of recreational ecstasy (MDMA).
-
PAUL A T KELLY
JNNP 2000 68: 690.[Full Text]