by Erowid
The Syrian Rue plant (seeds, roots, etc) contain a variety of alkaloids, the most prominent of which are the harmala alkaloids: harmine, harmaline, and tetrahydroharmaline. According to Jonathan Ott, surveys done on the plant material have shown the B-carboline alkaloids range from 2-7% of the dry weight of the mature seeds with harmine making up the largest part of this.
1841 - German chemist H. Goebel first isolates and names harmaline in an extract from Peganum harmala.
1847 - German chemist J. Fritzsche first isolates and names harmine from P. harmala.
1901 - Fischer isolates and names harmalol from P. harmala.
1980 - Allen & Holmstedt publish the presence of harmol, ruine, dihydroruine, and tetrahydroharmine in P. harmala seeds.
TiHKAL Entries:
RELATED VAULTS
Ayahuasca (brew)
Syrian Rue
Banisteriopsis Caapi
Harmala Vault
MAOI Vault
MERCK INDEX ENTRIES
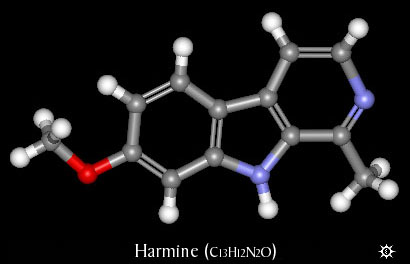
4647. Harmine. 7-Methoxy-1-methyl-9H-pyrido[3,4-b]indole; banisterine; yageine; telepathine; leucoharmine.
1841 - German chemist H. Goebel first isolates and names harmaline in an extract from Peganum harmala.
1847 - German chemist J. Fritzsche first isolates and names harmine from P. harmala.
1901 - Fischer isolates and names harmalol from P. harmala.
1980 - Allen & Holmstedt publish the presence of harmol, ruine, dihydroruine, and tetrahydroharmine in P. harmala seeds.
TiHKAL Entries:
| NAME : | Harmine |
| CHEMICAL NAME : | 7-Methoxy-1-methyl-9H-pyrido[3,4-b]indole |
| ALTERNATE CHEMICAL NAMES : | 7-Methoxy-1-methylpyrido[3,4-b]indole |
| ALTERNATE CHEMICAL NAMES : | banisterine; yageine; telepathine; leucoharmine. |
| CAS# | 442-51-3 |
| CHEMICAL FORMULA | C13H12N2O |
| MOLECULAR WEIGHT | 212.25 |
| MELTING POINT | 262° C (dec) - (Hydrochloride dihydrate crystals) |
| MELTING POINT | 321° C (anhydrous) - (Hydrochloride dihydrate crystals) |
| SOLUBILITY | freely soluble in hot water |
| OTHER | Flouresces in solution |
| LD50 | 38 mg/kg i.v.(mice) |
| From the Merck Index 12th Edition | |
|---|---|

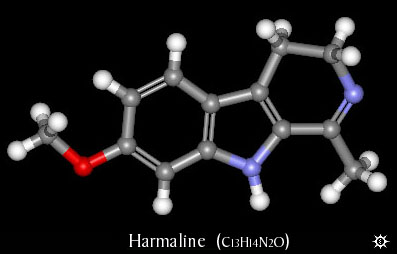
| NAME : | Harmaline |
| CHEMICAL NAME : | 4,9-Dihydro-7-methoxy-1-methyl-3H-pyrido[3,4-b]indole |
| ALTERNATE CHEMICAL NAMES : | 1-methyl-7-methoxy-3,4-dihydro-.beta.-carboline O-methylharmalol |
| ALTERNATE CHEMICAL NAMES : | 3,4-dihydroharmine; harmidine; harmalol methyl ether; O-methylharmalol |
| CAS# | 304-21-2 |
| CHEMICAL FORMULA | C13H14N2O |
| MOLECULAR WEIGHT | 216.279 |
| MELTING POINT | 173-176° C (plates from methanol) |
| MELTING POINT | 204-205° C (N-Acetylharmaline) - (needles) |
| SOLUBILITY | freely soluble in hot water |
| OTHER | Flouresces in solution |
| From the Merck Index 12th Edition | |
|---|---|

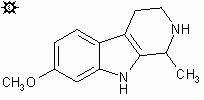
| NAME : | Tetrahydroharmine |
| CHEMICAL NAME : | 7-Methoxy-1,2,3,4-Tetrahydro-Harmine |
| ALTERNATE CHEMICAL NAMES : | Harman, 1,2,3,4-Tetrahydro-B-Carboline, 7-Methoxy-1-Methyl-1,2,3,4-Tetrahydro |
| ALTERNATE CHEMICAL NAMES : | 7-Methoxy-1,2,3,4-Tetrahydroharman; 1,2,3,4-Tetrahydroharmine; |
| ALTERNATE CHEMICAL NAMES : | 7-Methoxy-1-Methyl-1,2,3,4-Tetrahydro-B-Carboline; 7-Meo-Thh; leptaflorine |
| CHEMICAL FORMULA | C13H16N2O4P |
| MOLECULAR WEIGHT | 216.279 |
| MELTING POINT | 232-234#176; C (TiHKAL) |

RELATED VAULTS
Ayahuasca (brew)
Syrian Rue
Banisteriopsis Caapi
Harmala Vault
MAOI Vault
MERCK INDEX ENTRIES
4647. Harmine. 7-Methoxy-1-methyl-9H-pyrido[3,4-b]indole; banisterine; yageine; telepathine; leucoharmine.
- C13H12N2O; mol wt 212.25. C 73.57%, H 5.70%, N 13.20%, O 7.54%. CNS stimulant isolated from seeds
of Peganum harmala L., Zygophyllaceae: Gobel, Ann. 38, 363 (1841); Reinhard et al., Phytochemistry 7, 503 (1968); from Banisteria caapi Spruce, Malpighiaceae: Hochstein, Paradies, J. Am. Chem. Soc. 79,
5735 (1957); from Banisteriopsis inebrians Morton, Malpighiaceae: O'Connell, Lynn, J. Am. Pharm. Assoc. 42, 753 (1953). Structure: Manske et al., J. Chem. Soc. 1927, 1, 240. Synthesis: Spth,
Lederer, Ber. 63B, 120 (1930); Akaboro, Saito, ibid. 2245; Hahn et al., Ber. 67B, 2031 (1934), Ann. 520, 107, 123 (1935), Ber. 71B, 2163, 2175 (1938); Harvey, Robson, J. Chem. Soc. 1938, 97. Metabolism:
Slotkin, DiStefano, J. Pharmacol. Exp. Ther. 174, 456 (1970). Toxicity study: K. K. Chen et al., J. Pharmacol. Exp. Ther. 79, 127 (1943).
Slender, orthorhombic prisms from methanol, mp 261 deg (dec). Sublimes. pKa 7.70. uv max (methanol): 241, 301, 336 nm (log .epsilon. 4.61, 4.21, 3.69). Absorption and fluorescence spectra: O. S. Wolfbeis, E. Furlinger, Z. Physikal. Chem. 129, 171 (1982). Slightly sol in water, alcohol, chloroform, ether.
Hydrochloride dihydrate, crystals, mp 262 deg (dec), mp 321 deg when anhydrous. Sol in 40 parts water, freely sol in hot water. Aq solns have blue fluorescence. LD50 i.v. in mice: 38 mg/kg (Chen).
- C13H14N2O; mol wt 214.27. C 72.87%, H 6.59%, N 13.07%, O 7.47%. CNS stimulant from seeds of Peganum harmala L., Zygophyllaceae: Goebel, Ann. 38, 363 (1841); from Banisteria caapi Spruce, Malpighiaceae: Hochstein,
Paradies, J. Am. Chem. Soc. 79, 5735 (1957). Structure: Manske et al., J. Chem. Soc. 1927, 1. Synthesis: Spth, Lederer, Ber. 63, 120, 2102 (1930); Spenser, Can. J. Chem. 37, 1851 (1959). Identity with
harmidine: Robinson, Chem. Ind. (London) 1965, 605. Pharmacology: Fuentes, Longo, Neuropharmacology 10, 15 (1971). Metabolism: Ho et al., Biochem. Pharmacol. 20, 1313 (1971). Review of
structure and synthesis work: Hofmann, Sv. Farm. Tidskr. 75, 933 (1971), C.A. 76, 149609g (1972).
Orthorhombic bipyramidal prisms, tablets from methanol, rhombic octahedra from ethanol, mp 229-231 deg. Solns fluoresce blue. pK 4.2. uv max (methanol): 218, 260, 376 nm (log .epsilon. 4.27, 3.90, 4.02). Slightly sol in water, alcohol, ether; quite sol in hot alcohol, dil acids.
Hydrochloride dihydrate, slender, yellow needles, moderately sol in water, alcohol.
N-Acetylharmaline, needles, mp 204-205 deg.
- Ott, J. Pharmacotheon, 1996. Page 205.
- Kutlu H, Amal H, 1967. "Turkiyede yetisen Peganum harmala L. uzerinde kimyasal arastirmalar" Istanbul Universitesi Eczacilik Fakultesi Mecmuasi 3: 133-147. (from Ott)
- Flattery D, Schwartz M. Hoama and Harmaline 1989.


