The Tryptamine FAQ
v 1.9 May 20 2014 (v1.0 - 1991, last major update Jun 1999)
Originally published on Usenet
Citation: Pennanen P, Melbourne M. "The Tryptamine FAQ". Erowid.org. May 20 2014 (v1.0 - 1991, last major update Jun 1999). Online edition: Erowid.org/psychoactives/faqs/faqs_tryptamine.shtml
Erowid Note: This FAQ was not authored by Erowid. It may include out-of-date and/or incorrect information. Please check the version date to see when it was most recently revised. It appears on Erowid as part of our historical archives. For current information, see Erowid's summary pages in the substance's main vault.
Contents
Document homepage tryp.netThanks to many individuals for help in putting this together! If you know sources of tryptamines that are not mentioned here or have other comments please mail me.
A list of known or possibly psychoactive tryptamines.
MAO is the main enzyme that breaks down tryptamines in humans.
Enzymes in our bodies also construct tryptamines.
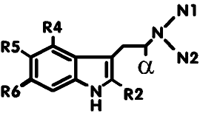
Psychotropic Tryptamine Derivatives |
|
| Name of Compound | R2 | N1 | N2 | alpha | R4 | R5 | R6 | Dosage (mg) |
Route | Duration (hr) |
|---|---|---|---|---|---|---|---|---|---|---|
| Typical effects in mentioned dose range | ||||||||||
| tryptamine | H | H | H | H | H | H | H | 250 | i.v. | short |
| Severe physical symptoms | ||||||||||
| DMT (dimethyltryptamine) (@ Deoxy, @ Erowid, @ Lycaeum) |
H | CH3 | CH3 | H | H | H | H | 30-100 | par | up to 1 |
| Intense psychedelic and hallucinogenic | ||||||||||
| 2-Me-DMT | CH4 | CH3 | CH3 | H | H | H | H | 50-100 | oral | 4-6 |
| Tactile sensitivity increased, sound distortion | ||||||||||
| 4-HO-DMT, psilocin | H | CH3 | CH3 | H | OH | H | H | 10-20 | oral | 3-6 |
| Psychedelic, more earth-bound and less abstract than LSD. Not a stimulant. Phosphate ester is psilocybin. | ||||||||||
| 5-HO-DMT, bufotenine | H | CH3 | CH3 | H | H | OH | H | 8-16 | i.v. | 1-2 |
| Severe physical symptoms. | ||||||||||
| 5-MeO-DMT | H | CH3 | CH3 | H | H | OCH3 | H | 5-20 | par | 1-2 |
| Intense psychedelic, less visual than DMT | ||||||||||
| 5-MeS-DMT | H | CH3 | CH3 | H | H | SCH3 | H | 15-30 | par | <1 |
| Stoned | ||||||||||
| 4,5-MDO-DMT | H | CH3 | CH3 | H | MeO2 | H | ? | ? | ? | |
| Unknown | ||||||||||
| 5,6-MDO-DMT | H | CH3 | CH3 | H | H | MeO2 | >5 | ? | ? | |
| Unknown | ||||||||||
| 5-MeO-TMT | CH3 | CH3 | CH3 | H | H | OCH3 | H | 75-150 | oral | 5-10 |
| Sense-enhancing psychedelic, significant physical side-effects at high doses | ||||||||||
| 4-HO-MET | H | CH3 | C2H5 | H | OH | H | H | 15-30 | par | <1 |
| Similar to psilocin | ||||||||||
| DET (@ Erowid) | H | C2H5 | C2H5 | H | H | H | H | 50-100 | oral | 2-4 |
| Psychedelic | ||||||||||
| 2-Me-DET | CH3 | C2H5 | C2H5 | H | H | H | H | 80-120 | oral | 6-8 |
| Sound distortion, more cloudy than 2-Me-DMT | ||||||||||
| 4-HO-DET, CZ-74 | H | C2H5 | C2H5 | H | OH | H | H | 10-25 | oral | 4-6 |
| Psychedelic with significant physical side-effects at high doses. | ||||||||||
| 5-MeO-DET | H | C2H5 | C2H5 | H | H | OCH3 | H | 1-3 | oral | 3-4 |
| Sense-enhancing psychedelic with heavy physical symptoms | ||||||||||
| 6-HO-DET | H | C2H5 | C2H5 | H | H | H | OH | 10 | i.m. | 4 |
| DET-like effects in one report | ||||||||||
| DPT (@ Erowid) | H | n-prop | n-prop | H | H | H | H | 60-250 | oral | 2-4 |
| Psychedelic with significant physical side-effects at high doses | ||||||||||
| 4-HO-DPT | H | n-prop | n-prop | H | OH | H | H | >20? | oral? | ? |
| Unknown | ||||||||||
| DIPT | H | i-prop | i-prop | H | H | H | H | 25-100 | oral | 6-8 |
| Auditory distortion and -hallucinations | ||||||||||
| 4-HO-DIPT | H | i-prop | i-prop | H | OH | H | H | 15-20 | oral | 2-3 |
| Intense and dose-sensitive psychedelic, mild physical symptoms | ||||||||||
| 5-MeO-DIPT (@ Erowid) | H | i-prop | i-prop | H | H | OCH3 | H | 6-12 | oral | 4-8 |
| Sense-enhancing psychedelic with some auditory distortion | ||||||||||
| 4,5-MDO-DIPT | H | i-prop | i-prop | H | MeO2 | H | >25 | oral | ? | |
| Psychedelic | ||||||||||
| 5,6-MDO-DIPT | H | i-prop | i-prop | H | H | MeO2 | ? | oral? | ? | |
| Unknown | ||||||||||
| MIPT (@ Erowid) | H | i-prop | CH3 | H | H | H | H | 10-25 | oral | 3-4 |
| Stimulating, sense-enhancing psychedelic | ||||||||||
| 4-HO-MIPT | H | i-prop | CH3 | H | OH | H | H | 6 | oral | ? |
| Sense-enhancing psychedelic, not stimulating | ||||||||||
| 4-MeO-MIPT | H | CH3 | H | H | OCH3 | H | H | 20-30 | oral | 4-6 |
| Sense-enhancing psychedelic | ||||||||||
| 5-MeO-MIPT | H | CH3 | H | H | H | OCH3 | H | 4-6 | oral | 4-6 |
| Sense-enhancing psychedelic | ||||||||||
| 5,6-MDO-MIPT | H | CH3 | H | H | H | MeO2 | >50 | oral | ? | |
| Some light-headedness at 75 mg | ||||||||||
| EIPT | H | i-prop | C2H5 | H | H | H | H | 24-40 | oral | 4-6 |
| Slightly psychedelic with dysphoric components and nausea | ||||||||||
| alpha,N-DMT | H | CH3 | H | CH3 | H | H | H | 50-100 | oral | 6-8 |
| Some amphetamine-like lightheadedness, loss of appetite | ||||||||||
| alpha,N,O-TMS 5-MeO-alpha,N-DMT |
H | CH3 | H | CH3 | H | OCH3 | H | 10-20 | oral | 6-8 |
| Somewhat uninspiring psychedelic | ||||||||||
| MBT | H | C4H9 | CH3 | H | H | H | H | 250-400 | oral | 4-6 |
| Slightly hallucinogenic with heavy physical symptoms | ||||||||||
| AMT, IT-290 (@ Erowid) | H | H | H | CH3 | H | H | H | 15-30 | oral | 12-16 |
| Stimulating psychedelic with little visuals and significant physical symptoms at high doses | ||||||||||
| 4-HO-AMT | H | H | H | CH3 | OH | H | H | 20 | oral | ? |
| Dizzy hallucinogen severe physical symptoms | ||||||||||
| 5-F-AMT | H | H | H | CH3 | H | F | H | 25 | oral | ? |
| Potent MAOI, an antidepressant rather than a psychedelic. | ||||||||||
| 5-MeO-AMT | H | H | H | CH3 | H | OCH3 | H | 2.5-4.5 | oral | 12-18 |
| Psychedelic, slight nausea or diarrhea at about 1 hour | ||||||||||
| AET | H | H | H | CH2CH3 | H | H | H | 100-150 | oral | 6-8 |
| Euphoric entactogenesis. Potential treatment for opiate withdrawal. | ||||||||||
| 5-MeO-AET | H | H | H | CH2CH3 | H | OCH3 | H | 50-75 | oral | ? |
| Some psychedelia with stimulant effects | ||||||||||
| serotonin | H | H | H | H | H | OH | H | 100 | oral | ? |
| Neurotransmitter, does not pass the blood-brain-barrier. Cardiovascular and autonomic symptoms. | ||||||||||
| pyr-T | H | tetraMe | H | H | H | H | ? | oral | ? | |
| Dizzy psychedelia with severe physical symptoms | ||||||||||
| 5-MeO-pyr-T | H | tetraMe | H | H | OCH3 | H | 0.5-2 | oral | several | |
| Severe physical symptoms, anaesthesia | ||||||||||
| 2,alpha-DMT | CH3 | H | H | CH3 | H | H | H | 300-500 | oral | 7-10 |
| Subtle psychedelia, not a stimulant | ||||||||||
| NPT | H | H | propyl | H | H | H | H | ? | ? | ? |
| Unknown, also holds for the i-propyl version (NIPT) | ||||||||||
| NBT | H | H | butyl | H | H | H | H | ? | ? | ? |
| Unknown, also holds for the i-butyl version (NIBT) | ||||||||||
| NSBT | H | H | s-butyl | H | H | H | H | 25-75 | oral | short |
| Generalized and diffuse intoxication | ||||||||||
| NTBT | H | H | t-butyl | H | H | H | H | 5-20 | oral | ? |
| Light headed, pleasant intoxication (similarities to GHB) | ||||||||||
| 4,alpha-DMT, MP 809 | H | H | H | CH3 | H | CH3 | H | 60 | oral | ? |
| An antidepressant rather than a psychedelic | ||||||||||
| 5,alpha-DMT | H | H | H | CH3 | H | CH3 | H | ? | ? | ? |
| Unknown | ||||||||||
| melatonin | H | acetyl | H | H | H | OCH3 | H | 0.5-75 | oral | - |
| Induces sleep, resets circadian rhythm. An antioxidant. | ||||||||||
- Other potentially psychedelic tryptamines include:
- 6-F-AMT, 7-methyltryptamine, 5-methyltryptamine, 5-fluorotryptamine,
6-fluorotryptamine and 5- and 6-fluorotryptophans.
MAO inhibitors and tryptamines
Monoamine oxidase (MAO) is the primary inactivation pathway of most tryptamines. That's why inhibitors of the MAO enzyme (MAOIs) can be used to potentiate the effects of tryptamines and to make DMT and 5-MeO-DMT orally active.MAO inhibitors fall into two classes: Irreversible and reversible MAOIs. In addition they can inhibit either or both of the two types of the MAO enzyme, MAO-A and MAO-B which are associated with serotonergic and dopaminergic neurons respectively. Irreversible MAOIs (e.g. the hydrazides iproniazid and phenelzine) bind permanently to the enzyme and cause MAO inhibition lasting 1-2 weeks after ingestion. They are used clinically to treat depression. Reversible MAOIs, such as moclobemide, which is used as an antidepressant, and the beta-carbolines harmine and harmaline, are effective for much shorter time, maybe up to 24 hours. Recreational drug users around the world are using mainly harmine and harmaline despite the lack of scientific studies on their effects on humans.
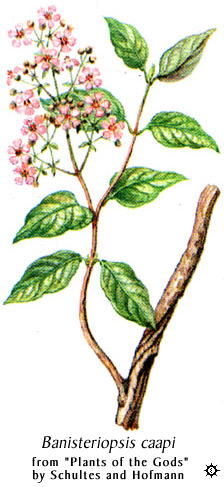 Natives of Amazon have traditionally combined Banisteriopsis caapi vine,
which contains harmine, harmaline and related beta-carbolines, with DMT-containing
plants to make an orally active brew called ayahuasca
(@ Erowid). Other
plants containing harmine and/or harmaline can be substituted for B. caapi. The usual 'North American ayahuasca'
consists of Peganum harmala (
picture, another picture,
seeds and Desmanthus illinoensis roots, and in
Australian 'acaciahuasca'
leaves of Acacia complanata are combined with material from
DMT-containing acacias (the effectivity of this mixture hasn't been
confirmed). Nausea is minimized in 'pharmahuasca', which consists of
pure DMT and a reversible MAO-inhibitor such as harmine or moclobemide.
When the DMT is in a plant extract taking it with a pure MAO inhibiting
substance instead of a plant containing MAOIs usually reduces nausea
and other body load significantly.
MAOIs have also been used to potentiate the effects of mushrooms containing
psilocybin and even phenethylamines -- the latter combination can be very
dangerous. DPT ingested with MAOIs has been called 'propylhuasca'; DPT
is orally active by itself and its dosage should be smaller when potentiated
by MAOIs.
Natives of Amazon have traditionally combined Banisteriopsis caapi vine,
which contains harmine, harmaline and related beta-carbolines, with DMT-containing
plants to make an orally active brew called ayahuasca
(@ Erowid). Other
plants containing harmine and/or harmaline can be substituted for B. caapi. The usual 'North American ayahuasca'
consists of Peganum harmala (
picture, another picture,
seeds and Desmanthus illinoensis roots, and in
Australian 'acaciahuasca'
leaves of Acacia complanata are combined with material from
DMT-containing acacias (the effectivity of this mixture hasn't been
confirmed). Nausea is minimized in 'pharmahuasca', which consists of
pure DMT and a reversible MAO-inhibitor such as harmine or moclobemide.
When the DMT is in a plant extract taking it with a pure MAO inhibiting
substance instead of a plant containing MAOIs usually reduces nausea
and other body load significantly.
MAOIs have also been used to potentiate the effects of mushrooms containing
psilocybin and even phenethylamines -- the latter combination can be very
dangerous. DPT ingested with MAOIs has been called 'propylhuasca'; DPT
is orally active by itself and its dosage should be smaller when potentiated
by MAOIs.
 Peganum harmala (Syrian rue) seeds - pictured - are the most concentrated natural source
of harmine and harmaline - about 3% of their weight consists of these
alkaloids. Banisteriopsis caapi has been found to contain from 0.18% to
1.36% beta-carbolines, with the concentration of harmine being from 0.057%
to 0.635% (McKenna et al. 1984). According to anecdotal
reports one gram (corresponding to approx. 30 mg of harmine/harmaline)
of P. harmala seeds ingested inhibits MAO enough to make DMT orally active,
while 75 mg of moclobemide is sufficient for the same purpose.
Peganum harmala (Syrian rue) seeds - pictured - are the most concentrated natural source
of harmine and harmaline - about 3% of their weight consists of these
alkaloids. Banisteriopsis caapi has been found to contain from 0.18% to
1.36% beta-carbolines, with the concentration of harmine being from 0.057%
to 0.635% (McKenna et al. 1984). According to anecdotal
reports one gram (corresponding to approx. 30 mg of harmine/harmaline)
of P. harmala seeds ingested inhibits MAO enough to make DMT orally active,
while 75 mg of moclobemide is sufficient for the same purpose.
Harmine and harmaline are hallucinogenic on their own with doses starting from around 300 mg (Naranjo 1967), but often cause physical side-effects such as nausea and tremors in this dose range. They have little emotional or 'psychedelic' effects, but produce strong visual hallucinations. Because of this the natives of Amazon often add larger amounts (75-100 cm of stem per dose) of B. caapi to ayahuasca brew than is needed for MAO inhibition (Luna 1984). The psychedelic effects of moclobemide at normal antidepressant doses (e.g. 150 mg/day) are limited to slight hallucinations of the sense of balance, which is rarely affected by other drugs.
A combination of a MAO inhitor and DMT tends to induce drowsiness. Perhaps this is why natives often smoke tobacco and/or drink caffeine-containing drinks while preparing and tripping on ayahuasca. The MAO-B inhibition of nicotine might also be a reason for smoking it, although it is unclear to which extent inhibition of just MAO-B activates or potentiates tryptamines.
There are significant dangers in using MAO inhibitors. Most MAOIs potentiate the cardiovascular effects of tyramine and other monoamines found in foods. Ingestion of aged cheese, beer, wine, pickled herring, chicken liver, yeast, large amounts of coffee, citrus fruits, canned figs, broad beans, chocolate or cream while MAO is inhibited can cause a hypertensive crisis including a dangerous rise in blood pressure. You can also look at a more complete list of foods to avoid when receiving MAOI therapy. MAOIs interact with other psychoactive substances in addition to tryptamines; effects of amphetamines, general anaesthetics, sedatives, anti-histamines, alcohol, potent analgesics and anticholinergic and antidepressant agents are prolonged and intensified. Overdosage of MAOIs by themselves is also possible with effects including hyperreflexia and convulsions. It should be noted, however, that these strict warnings apply best in the case of irreversible MAOIs, and many users of reversible MAOIs to activate or potentiate tryptamines are not quite as careful with no apparent ill effects.
Self-synthesis of DMT Derivatives
Tryptamine derivatives and beta-Carbolines have been detected as endogenous metabolites in mammals, including humans. Methyl transferases that catalyze the synthesis of tryptamines, including DMT, 5-MeO-DMT and bufotenine, are found in human lung, brain, cerebrospinal fluid, liver and heart (McKenna & Towers 1984). In the pineal gland MAO is the primary inactivation pathway of serotonin, a neurotransmitter synthesized from the amino acid tryptophan. If MAO is blocked by harmine, harmaline or other MAO inhibitors serotonin can be converted by the methyltransferase enzymes HIOMT and INMT into psychedelic tryptamines (serotonin --(HIOMT)--> 5-MeO-trypt. --(2*INMT)--> 5-MeO-DMT).So, ingesting l-tryptophan to increase serotonin levels, a candy bar to increase the amount of tryptophan getting to your brain and natural plant material containing 25-50 mg harmine/harmaline to block MAO, all at the same time, might cause your pineal gland to synthesize substantial amounts of 5-MeO-DMT (Most 1986). Similar results might be obtained by substituting 5-hydroxytryptophan (5-HTP) for tryptophan. Normal sleep-inducing doses of melatonin have also been taken with reversible MAOIs with the resulting psychoactive effects suggesting significant interaction of the two substances. This is extremely dangerous for persons with existing amine imbalance or schizophrenia. For normal, healthy people possible consequences are bad.
A potent inhibitor of INMT, which is a necessary enzyme for the synthesis of DMT and 5-MeO-DMT, is found in particularly high concentrations in the pineal gland. A bypassing or inhibition of the synthesis of this inhibitor might be responsible for trances and other psychedelic states achieved "without drugs" (Strassman 1990). See Strassman's article for more info and speculation about the pineal gland.
The Animals
Many animals, including mammals, contain psychoactive tryptamines. Below are two examples.
- Family: Hominidae
- Genus: Homo
- Species: sapiens
- Genus: Bufo
- Species: alvarius
 Bufotenine and related 5-hydroxy-indolethylamines are common constituents
of venoms of the genera Hyla, Leptodactylus, Rana and Bufo. Bufotenine
is not psychedelic in reasonable doses (with larger doses there are
dangerous physiological side effects), but the skin of one species, Bufo
alvarius, contains 50-160 mg 5-MeO-DMT/g of skin (Daly & Witkop 1971).
It's a very large (upto 17 cm) toad with cranial crests and elongated
parotid glands, and is the only Bufo species known to contain a hallucinogenic tryptamine
(McKenna & Towers 1984). Most (1984) gives instructions for collecting
and drying the venom:
Bufotenine and related 5-hydroxy-indolethylamines are common constituents
of venoms of the genera Hyla, Leptodactylus, Rana and Bufo. Bufotenine
is not psychedelic in reasonable doses (with larger doses there are
dangerous physiological side effects), but the skin of one species, Bufo
alvarius, contains 50-160 mg 5-MeO-DMT/g of skin (Daly & Witkop 1971).
It's a very large (upto 17 cm) toad with cranial crests and elongated
parotid glands, and is the only Bufo species known to contain a hallucinogenic tryptamine
(McKenna & Towers 1984). Most (1984) gives instructions for collecting
and drying the venom:
Fresh venom can easily be collected without harm to the toad. Use a flat glass plate or any other smooth, nonporous surface at least 12-inches square. Hold the toad in front of the plate, which is fixed in a vertical position. In this manner, the venom can be collected on the glass plate, free of dirt and liquid released when the toad is handled.Davis and Weil (1992) smoked the venom and described what happened:
When you are ready to begin, hold the toad firmly with one hand and, with the thumb and forefinger of your other hand, squeeze near the base of the gland until the venom squirts out of the pores and onto the glass plate. Use this method to systematically collect the venom from each of the toad's granular glands: those on the forearm, those on the tibia and femur of the hind leg, and, of course, the parotids on the neck. Each gland can be squeezed a second time for an additional yield of venom if you allow the toad a one-hour rest preiod. After this the glands are empty and require four to to six weeks for regeneration.
The venom is viscous and milky-white in color when first squeezed from the glands. It begins to dry within minutes and acquires the color and texture of rubber cement. Scrape the venom from the glass plate, dry it thoroughly, and store it in an airtight container until you are ready to smoke it.
In comparison to the pure compounds the toad venom appears longer lasting and, because one does not completely lose contact with reality, far more pleasant, even sensual. Shortly after inhalation I experienced warm flushing sensations, a sense of wonder and well-being, strong auditory hallucinations, which included an insect-cicada sound that ran across my mind and seemed to link my body to the earth. Though I was indoors, there was a sense of the feel of the earth, the dry desert soil passing through my fingers, the stars at midday, the scent of cactus and sage, the feel of dry leaves through hands. Strong visual hallucinations in orblike brilliance, diamond patterns that undulated across my visual field. The experience was in every sense pleasant, with no disturbing physical symptoms, no nausea, perhaps a slight sense of increased heart rate. Warm waves coursed up and down my body. The effects lasted only a few minutes but a pleasant afterglow continued for almost an hour. (Wade Davis, personal observation, January 12, 1991)Other animals contain DMT such as the gorgonian Paramuricea chamaeleon (Cimino & De Stefano, 1978).
Profound alteration of consciousness within a few seconds of exhaling. I relax into a deep, peaceful interior awareness. There is nothing scary about the effects and no sense of toxicity. I try to describe my feelings but am unable to talk for the first five minutes and then only with some difficulty. This is a powerful psychoactive drug, one that I think would appeal to most people who like the effects of hallucinogens. For the next hour I feel slow and velvety, with a slight pressure in my head. No long-lasting effects to report. (Andrew T. Weil, personal observation, January 12, 1991).
 |
he Fungi |
The psychoactive tryptamines in fungi are mainly psilocybin and psilocin although others are also present. Paul Stamets has compiled a comparison of combined maximum percentages of psilocybin, psilocin and baeocystin in 11 species of Psilocybe.
- Family: Bolbitiaceae
- Genus: Agrocybe
- Species: farinacea
- Genus: Conocybe
- Species: cyanopus, kuehneriana, siligineoides, smithii
- Family: Coprinaceae
- Genus: Copelandia
- Species: anomala, bispora, cambodginiensis, chlorocystis, cyanescens, tropicalis
- Genus: Panaeolina,
- Species: castaneifolius, foenisecii
- Genus: Panaeolus
- Species: africanus, antillarum, ater, campanulatus, firmicola, microsporus, olivacens, retirugis, separatus, sphinctrinus, subbalteatus
- Genus: Psathyrella
- Species: candollenana
- Family: Cortinariaceae
- Genus: Galerina
- Species: steglichii
- Genus: Gymnopilus
- Species: aeruginosus, liquiritiae, luteus, purpuratus, spectabilis, validipes, viridans
- Genus: Inocybe
- Species: aeruginascens, calamistrata [Erowid Note: Alan Rockefeller states that I. calamistrata was a false positive and does not contain psilocybin-type chemicals.], coelestium, corydalna, haemacta, tricolor
- Family: Lepiotaceae
- Genus: Lepiota
- Species: humei Murrill also known as peele "Peele's Lepiota"
- Family: Pluteaceae
- Genus: Pluteus
- Species: atricapillus, cyanopus, glaucus, nigroviridis, salicinus
- Family: Polyporaceae
- Genus: Gerronema
- Species: fibula, swartzii
- Genus: Hygrocybe
- Species: psittacina

- Family: Strophariaceae
- Genus: Psilocybe (@ erowid)
- Species: 75 Known hallucinogenic species such as cubensis (pictured)+ antioquensis, aucklandii, beliconiae, coprophila, crobulus, guatapensis, makarorae, samuiensis, subacutipilea
Images of Psilocybe azyrescens, baeocystis, cubensis, cyanescens, cyanofibrillosa, azurescens, pelliculosa, semilanceata, silvatica, stuntzii and weilii can be seen at A Gallery of Psilocybe Mushrooms
 |
The Plants |
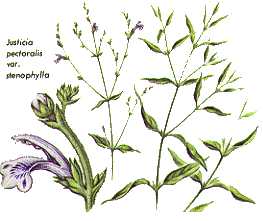
- Family: Acanthaceae
- Genus: Justicia
- Species: pectoralis (var. stenophylla)
- Family: Aizoaceae
- Genus: Delosperma
- Family: Alariaceae
- Genus: Ecklonia
- Species: maxima
- Family: Apocynaceae
- Genus: Prestonia
- Species: amazonica?
- Family: Cactaceae
- Genus: Echinocereus
- Species: salm-dyckianus, triglochidiatus
- Genus: Trichocereus
- Species: terscheckii "Cardon grande"
- Family: Caesalpininaceae
- Genus: Petalostylis
- Species: cassiodies
- Family: Fabaceae
- Genus: Desmodium
- Species: gangetium, gyrans, tiliaefolium, triflorum,
- Genus: Lespedeza
- Species: bicolor
- Genus: Mucuna
- Species: pruriens
- Genus: Phyllodium
- Species: pulchellum
- Family: Mimosaceae
- Genus: Anadenanthera (Piptadenia)
- Species: colubrina (picture @ Erowid) contorta, excelsa, macrocarpa, peregrina
The trees grow in open plain areas, and leaves, bark and seeds contain DMT, 5-MeO-DMT and related compounds (Schultes 1976, 1977; Pachter et al. 1959; Phytochem 11, 737). Smoking half a gram of A. colubrina seeds has been reported to give a mild psychedelic effect.

- Genus: Acacia
- Species: confusa, jurema, maidenii pictured (pictures @ Erowid), niopo, nubica, phlebophylla, polycantha subsp. campylacantha, senegal, simplex, tortilis
- Genus: Desmanthus
- Species: illinoensis pictured (pictures @ Erowid) "Illinois Bundleflower"
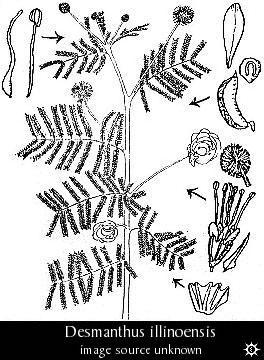 Thompson et al. report that the root bark of this North American perennial
shrub contains 0.34% DMT and 0.11% N-methyltryptamine. The bark accounts
for about a half of the total weight of the roots. The plant should be
resistant to cold and draught and easy to grow.
D. illinoensis seeds, dried root and dried root bark
can be ordered from several suppliers. Seeds are also available from more main-stream
mail-order houses.
Thompson et al. report that the root bark of this North American perennial
shrub contains 0.34% DMT and 0.11% N-methyltryptamine. The bark accounts
for about a half of the total weight of the roots. The plant should be
resistant to cold and draught and easy to grow.
D. illinoensis seeds, dried root and dried root bark
can be ordered from several suppliers. Seeds are also available from more main-stream
mail-order houses.
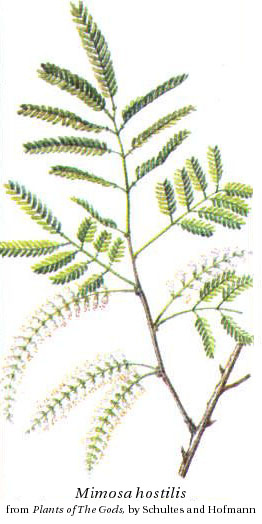 The roots of M. hostilis, which is not the common houseplant M. pudica
("sensitive plant"), contain 0.57% DMT and are used by Indians of Pernambuso
State in Brazil as part of their Yurema cult (Pachter et al. 1959,
Schultes 1977, (Meckes-Lozoya et al. 1990). The root bark can
be powdered e.g. in a coffee grinder, boiled in 30% lemon juice for an hour and strained through a
paper filter to produce liquid for ingestion. 8-20 g of the root bark prepared this way has been reported
to give a trip with nausea, both the intensity of the psychedelic effect and the nausea being strong at the
20 g dosage. An acid-base extraction method (involving e.g. hydrochloric acid, sodium hydroxide and petroleum ether
with CaCO2 for drying) can be used to get DMT crystals, which can then be smoked (below, a recipe for Phalaris).
As M. hostilis root bark is one of the most concentrated plant sources of DMT mild effects can be felt from smoking ground bark.
M. scabrella contains DMT and
N-methyltryptamine (De Moraes et al., 1990). Bark of M. verrucosa also contains DMT (Smith 1977).
The roots of M. hostilis, which is not the common houseplant M. pudica
("sensitive plant"), contain 0.57% DMT and are used by Indians of Pernambuso
State in Brazil as part of their Yurema cult (Pachter et al. 1959,
Schultes 1977, (Meckes-Lozoya et al. 1990). The root bark can
be powdered e.g. in a coffee grinder, boiled in 30% lemon juice for an hour and strained through a
paper filter to produce liquid for ingestion. 8-20 g of the root bark prepared this way has been reported
to give a trip with nausea, both the intensity of the psychedelic effect and the nausea being strong at the
20 g dosage. An acid-base extraction method (involving e.g. hydrochloric acid, sodium hydroxide and petroleum ether
with CaCO2 for drying) can be used to get DMT crystals, which can then be smoked (below, a recipe for Phalaris).
As M. hostilis root bark is one of the most concentrated plant sources of DMT mild effects can be felt from smoking ground bark.
M. scabrella contains DMT and
N-methyltryptamine (De Moraes et al., 1990). Bark of M. verrucosa also contains DMT (Smith 1977).
- Genus: Testulea
- Species: gabonensis
- Family: Malpighiaceae
- Genus: Banisteriopsis
- Species: muricata (=argentea), rusbyana (see Diplopterys cabrerana)
- Genus: Diplopterys
- Species: cabrerana
- Family: Myristicaceae
- Genus: Horsfieldia
- Species: superba
- Genus: Iryanthera
- Species: macrophylla

- Genus: Virola
- Species: calophylla, calophylloidea, rufula, sebifera, theiodora (pictured)
Amazonian Colombia natives roll small pellets of boiled resin in a evaporated filtrate of bark ashes of Gustavia Poeppigiana and ingest them to bring on a rapid intoxication (Smith 1977, Schultes 1977).
- Family: Pandanaceae
- Genus: Pandanus "Screw pine"
Leaves, flowers and rhizomes contain DMT, bufotenine and related compounds (Ghosal et al. 1972).

- Genus: Phalaris
- Species: aquatica (tuberosa) pictured, arundinacea
Jim DeKorne (1996) has reported that strong varieties of P. Arundinacea can be processed simply with a wheatgrass juicer to yield a liquid that is potent enough for one teaspoon to be effective with MAOIs. He has prepared a potent smokable extract as follows: grass clippings are pulverized and water is added to make a pourable mixture, which is then acidified to about pH 5. The mix can then be heated overnight while evaporation is not allowed. The plant matter is separated by first using a cheesecloth and then a paper coffee filter. 10-15% of the mass of the resulting solution of a "defatting solvent" (e.g. methylene chloride, ether, chloroform, or naptha) is then added. After vigorous shaking the water containing the alkaloids is separated from the solvent. A base is added to the aqueous solution in small increments until the pH gets to about 9 or 10, which converts the alkaloids into their free base. Extraction is then performed four times with an organic solvent, comprising 10% of the mass of the solution, first after 24 hours and then at three weekly intervals, while the solvent layer takes on a darker tint (usually yellowish or reddish-brown). In total the extraction takes almost a month and the solution should be shaken at least twice a day between extractions. To isolate the alkaloids the solvent is finally evaporated from the combined extract fractions.
- Genus: Phragmites
- Species: australis
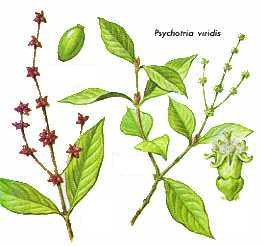
- Family: Rubiaceae
- Genus: Psychotria
- Species: carthaginensis, viridis (psychotriaefolia) pictured, picture 2, picture 3,
- Family: Rutaceae
- Genus: Dictyoloma
- Species: incanescens
- Genus: Limonia
- Species: acidissima
- Genus: Melicope
- Species: leptococca
- Genus: Vepris
- Species: ampody
- Genus: Zanthoxylum
- Species: aborescens
References
Abu Zarga, M.H. 1986. Three new simple indole alkaloids from Limonia acidissima. Lloydia 49(5), 901-904.
Akers, B.P. 1992. Peele's Lepiota: An identification and a clarification. Mycotaxon 43(0), 461-469.
Arora, D. 1986. Mushrooms Demystified: A Comprehensive Guide to the Fleshy Fungi. Ten Speed Press, Berkeley.
Arthur, H.R., Loo, S.N. & Lamberton, J.A. 1967. Nb-methylated tryptamines and other constituents of Acacia confusa Merr. of Hong Kong. Aust. J Chem. 20, 811.
Banerjee, P.K. & Ghosal, S. 1969. Simple indole bases of Desmodium gangeticum. Aust. J Chem. 22, 275-277.
Barrau, J. 1958.
Nouvelles observations au sujet des plantes hallucinogenes d'usage autochtone en Nouvelle-Guinee. J Agric. Trop. Bot. Appl. 5, 377-378.
Barrau, J. 1962.
Observations et travaux recents sur les vegetaux hallucinogenes de la Nouvelle-Guinee. J Agric. Trop. Bot. Appl. 9, 245-249.
Benedict, R.G., Brady, L.R., Smith, A.H. & Tyler, V.E. 1962.
Occurrence of psilocybin and psilocin in certain Conocybe and Psilocybe species. Lloydia 25, 156-159.
Benedict, R.G., Tyler, V.E. & Watling, R. 1967.
Blueing in Conocybe, Psilocybe and a Stropharia Species and the Dectection of Psilocybin. Lloydia 30(2), 150-157.
Besl, H. 1993.
Galerina steglichii spec. nov., a hallucinogenic Galerina. Zeitschrift fuer Mykologie 59(2), 215-218.
Bresinsky, A. & Besl, H. 1990.
A Colour Atlas of Poisonous Fungi. Wolfe Publishing Ltd, London.
Bye, R.A. 1979.
Hallucinogenic plants of the Tarahumara. J. Ethnopharmacology 1, 23-48.
Corbett, L., Christian, S.T., Morin, R.D., Benington, F. & Smythies, J.R. 1978.
Hallucinogenic N-methylated indolealkylamines in the cerebrospinal fluid of psychiatric and control populations. British J. Psychiatry 132, 139.
Christiansen, A.L., Rasmussen, K.E. & Hoeiland, K. 1984.
Detection of psilocybin and psilocin in Norwegian species of Pluteus and Conocybe. Planta Med. 50, 341-343.
Cimino, G. & De Stefano, S. 1978.
Chemistry of Mediterranean Gorgonians. Simple indole derivatives from Paramuricea chamaeleon. Comptes Rendus Biochem. Physiol. Ser. C. 61, 361-362.
Crouch, I.J., Smith M.T., Van Staden J., Lewis, M.J. & Hoad, G.V. 1992.
Identification of auxins in a commercial seaweed concentrate. J Plant Physiology 139(5), 590-594.
Daly, J.W. & Witkop, B. 1971.
Chemistry and pharmacology of frog venoms. In: Venomous animals and their venoms. Vol II. New York: Academic Press.
Davis, W. & Weil, A.T. 1992.
Identity of a New World Psychoactive Toad. Ancient Mesoamerica 3 (1992) 5, 51-59.
DeKorne, J. 1996
Ayahuasca analogs and Plant-Based Tryptamines.
De Moraes, E.H.F., Alvarenga, Z.M.A., Ferreira, Z.M.G.S. & Alisue, G. 1990.
Quim. Nova 13, 308.
Fitzgerald, J.S. & Sioumis, A.A. 1965.
Alkaloids of Australian Leguminosae V. Aust. J Chem. 18, 433.
Fiussello, N. & Ceruti-Scarti, J. 1971/72.
Presenza di psilocibina edi 5-idrossi-indolderivati in Panaeolus retirugis. Atti Acc. Sci. Torino 106, 725-735.
Gartz, J. 1991.
Influence of phosphate on fruiting and secondary metabolism of mycelia of Psilocybe cubensis, Psilocybe semilanceata and Gymnopilus purpuratus. Zeitschrift fuer Mykologie 57(1), 149-154.
Ghosal, S., Chaudhuri, R.K., Dutta, S.K. & Bhattacharya, S.K. 1972.
Occurrence of curaromimetic indoles in the flowers of Arundo donax. Planta Med. 21, 22.
Ghosal, S. & Mukherjee, B. 1966.
Indole-3-alkylamine Bases of Desmodium pulchellum. J, Org. Chem. 31, 2284.
Ghosal, S., Singh, S. & Bhattacharya, S.K. 1971.
Alkaloids of Mucuna pruriens, Chemistry and Pharmacology. Planta Med. 19, 279
Grina, J.A. et al. 1982.
Constituents of Zanthoxylum aborescens. Part 7. Old & new alkaloids from Zanthoxylum aborescens. J. Organic Chemistry 47, 2648-2651.
Gurevich, L.S. 1993.
Indole derivatives in certain Panaeolus species from East Europe & Siberia. Mycological Research 97(2), 251-254.
Gurevich, L.S. & Astapenko, V.V. 1992.
Chromatographic study of some indole metabolites in Panaeolus basidiomycetes. Mikologiya I Fitopathologiga 26(3), 189-194.
Guzman, G. 1983.
The Genus Psilocybe. Beihefte Zur Nova Hedwingia 74, 1-439.
Guzman, G., Bandala, V.M. & Allen, J.W. 1993.
A New Bluing Psilocybe from Thailand. Mycotaxon 26, 155-160.
Guzman, G., Bandala, V.M. & King, C. 1991.
A New Species of Psilocybe of Section Zapotecorum from New Zealand. Mycological Research 95, 507-508.
Guzman, G., Saldarriaga, Y., Pineda, F., Garcia, G. & Velazquez, L.F. 1994.
New Species of Psilocybe from Colombia and Discussion on the known species. Mycotaxon , 225.
Haeselbarth, G., Michaelis, H. & Salnikow, J. 1985.
Nachweis von Psilocybin in Inocybe aeruginescens. Mykol. Mitt. bl. 28(1), 59-62.
Hatfield, G.M., Valdes, L.J. & Smith, A.H. 1978.
The occurrence of psilocybin in Gymnopilus species. Lloydia 41, 140-144.
Hein, R. & Wasson, R.G. 1958.
Les champignons hallucinogenes du Mexique. Museum National d'Histoire Naturelle, Paris.
Holmstedt, B., Lindgren, J.E., et al. 1980.
Indole alkaloids in Amazonian Myristicaceae: Field and laboratory research. Bot. Mus. Leafl., Harvard Univ. 28, 215-234.
Johns, S.R., Lamberton, J.A. & Sioumis, A.A. 1966.
Alkaloids of the Australian Leguminosae VI. Aust. J Chem. 19, 893.
Johnston, P.R. & Buchanan, P.K. 1995.
The genus Psilocybe (Agaricales) in New Zealand. New Zealand Journal Botany 33(3), 379.
Jossang, A., Jossang, C., Hadi, H.A., Sevenet, T. & Bodo, B. 1991.
Horsfiline, an oxindole alkaloid from Horsfieldia superba. J. Organic Chem. 56(23), 6527-6530.
Kan Fan, C. et al. 1970.
Alcaloides de Vepris ampody (Rutacees). Phytochem. 9, 1283-1291.
Kantor, R.E., Dudlettes, S.D. & Shulgin, A.T. 1980.
5-Methoxy-alfa-methyl- tryptamine (alfa,O-dimethylserotonin), a hallucinogenic homolog of serotonin. Biological Psychiatry Vol 15, 349-352.
Koike, Y., Wada, K., Kusano, G., Nozoe, S., & Yokoyama, K. 1981.
Isolation of Psilocybin from Psilocybe argentipes and its Determination in Specimens of some Mushrooms. Lloydia 44(3), 362-365.
Leboeuf, M. et al., 1977.
Alkaloids and triterpenes of Testulea gabonensis. Plant Medicine Phytotherapy 11, 230.
Luna, L.E. 1984.
The Healing Practices of a Peruvian Shaman. J. Ethnopharmacology 11, 123-133.
McKenna, D.J., Towers, G.H.N., & Abbott, F. (1984).
Monoamine oxidase inhibitors in South American hallucinogenic plants: Tryptamines and Beta-carboline constituents of ayahuasca. J Ethnopharmacology 10, 195-223.
Mckenna, D.J. & Towers, G.H.N. 1984.
Biochemistry and Pharmacology of Tryptamines and beta-Carbolines: A Minireview. J Psychoactive Drugs 16(4).
Meckes-Lozoya, M., Lozoya, X., Marles, R.J., Soucy-Breau, C., Sen, A. & Arnason, J.T. 1990.
N,N-dimethyltryptamine alkaloid in Mimosa tenuiflora bark (tepescohuite). Arch. Invest. Med. Mex. 21(2), 175-7.
Merlin, M.D. & Allen, J.W. 1993.
Species identification and chemical analysis of psychoactive fungi in the Hawaiian islands. Journal of Ethnopharmacology 40(1), 21-40.
Miller Jr., O.K. 1972.
Mushrooms of North America. Dutton & Co., Springfield.
Most, Albert. 1984.
Bufo Alvarius: the Psychedelic Toad of the Sonoran Desert. Venom Press Box 2863 Denton TX 76202.
Most, Albert. 1986.
Eros and the Pineal: the layman's guide to cerebral solitaire. Venom Press, Denton, TX.
Naranjo, C. 1969.
Psychotropic Properties of the Harmala Alkaloids. In: Efron (Ed.) The Ethnopharmacologic Search for Psychoactive Drugs.
Ohenoja, E., Jokiranta, J., Makinen, T., Kaikkonen, A. & Airaksinen, M.M. 1987. The Occurrence of Psilocybin and Psilocin in Finnish Fungi. Lloydia 50(4), 741-744.
Ott, J. 1993. Pharmacotheon. Pangean Entheogens.
Ott, J. 1994. Ayahuasca Analogues. Natural Products Co..
Pachter, I.J, Zacharias, D.E & Ribeir, O. 1959.
Indole Alkaloids of Acer saccharinum (the Silever Maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis. J Org Chem 24, 1285-7.
Phillips, R. 1981.
Mushrooms and other fungi of Great Britain and Europe. Pan Books, London.
Poupat, C., Ahond, A. & Sevenet, T. 1976.
Alcaloides De Acaia simplicifolia. Phytochemistry 15, 2019-2120.
Rivier, L. & Lindgren, J-E. 1972.
"Ayahuasca," the South American Hallucinogenic Drink: an Ethnobotanical and Chemical Investigation. Economic Botany 26, 101-129.
Rivier, L. & Pilet, P.E. 1971.
Annee Biologique 10, 129.
Robbers, J.E., Tyler, V.E. & Ola'h, G.M. 1969.
Additional evidence supporting the occurrence of psilocybin in Panaeolus foenisecii. Lloydia 32, 399-400.
Rovelli, B. & Vaughan, G.N. 1967.
Alkaloids of Acacia I. Dimethyltryptamine in Acacia phlebophylla. Aust. J Chem. 20, 1299-1300.
Saupe, S.G. 1981.
Occurence of Psilocybin/Psilocin in Pluteus salicinus (Pluteaceae). Mycologia 73, 781-784.
Schultes, R.E. 1976.
Indole Alkaloids in Plant Hallucinogens. J of Psychedelic Drugs Vol 8(1), 7-25.
Schultes, R.E. 1977.
The Botanical and Chemical Distribution of Hallucinogens. J of Psychedelic Drugs Vol 9(3), 247-263.
Schultes, R.E. 1979.
Hallucinogenic Plants: Their Earliest Botanical Descriptions. J of Psychedelic Drugs Vol 11(1-2), 13-24.
Schultes, R.E. & Hofmann, A. 1979.
Plants of the Gods. McGraw-Hill. Reprint available from Healing Arts Press, Rochester, VT.
Schultes, R.E. & Hofmann, A. 1980.
The Botany and Chemistry of Hallucinogens. Springfield, Ill: Thomas.
Shulgin, A.T. 1976.
Psychotomimetic agents. In: Gordon, M. (Ed.) Psychopharmacological Agents, Vol IV. New York: Academic Press.
Shulgin, A.T. 1982.
Chemistry of Psychotomimetics. In: Hoffmeister, F. & Stille, G. (Eds.) Handbook of Experimental Pharmacology, Vol 55: Alcohol and Psychotomimetics, Psychotropic Effects of Central-Acting Drugs. New York: Springer-Verlag.
Shulgin, A.T. & Shulgin, A. 1997.
Tihkal -- The Continuation. Berkeley: Transform Press.
Skaltsounis, A.L., Tillequin, F. & Koch, M. 1983.
Plantes des Nouvelle- Caledonie LXXXIII: Alcaloides des tiges feuillees de Melicope leptococca. Lloydia 46(5), 732.
Smith, T.A. 1977.
Review: Tryptamine and Related Compounds in Plants. Phytochemistry 16 171-175.
Stein, S.I. 1959.
Clinical Observations on the Effects of Panaeolus venenosus Versus Psilocybe caerulescens Mushrooms. Mycologica 51, 49.
Stijve, T., Klan, J. & Kugper, W. 1985.
Occurrence of psilocybin and baeocystin in the genus Inocybe. Persoonia 12, 469-472.
Stijve, T. & De Meijer, A.A.R. 1993.
Macromycetes from the state of Parana, Brazil. 4. The psychoactive species. Arquivos de Biologia e Tecnologia (Curitiba) 36(2), 313-329.
Strassman, R.J. 1990.
The Pineal Gland: Current Evidence For Its Role In Consciousness. In: Lyttle, T. (Ed.) Psychedelic Monographs and Essays Vol 5.
Thompson, A.C., Nicollier, G.F. & Pope, D.F 1987.
Indolealkylamines of Desmanthus illinoensis and Their Growth Inhibition Activity. J Agric. Food Chem. 35, 361-365.
Wahba, S.K. & Elkheir, Y.M. 1975.
Dimethyltryptamine from the leaves of certain Acacia species of northern Sudan. Lloydia 38, 176-177.
Wassel, G.M. et al. 1985.
Alkaloids from the rhizomes of Phragmites australis Cav. Scientia Pharmaceutica 53, 169-170.
Suppliers
Credits
Images from© Petrus Pennanen 1999
* Everything is perfect forever [-P.P.]
* Ditto [-M.]