
#64 DOC
2,5-DIMETHOXY-4-CHLOROAMPHETAMINE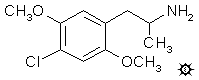
|
| [3D .mol structure] |
DOSAGE: 1.5 - 3.0 mg.
DURATION: 12 - 24 h.
QUALITATIVE COMMENTS: (with 1.6 mg) I was hit with a slightly light head; the effects were quite real. I was disconnected, and somehow spacey, but this was a favorable spacey which was kind of fun. Somewhere at about the sixth hour I realized that I was beginning to drop off a bit, but six hours later yet, there was still a lot of memory. This is a long thing.
(with 2.4 mg) This is what I might call an archetypical psychedelic. Everything is there in spades, with few if any of the subtle graces, the `gentle images' and `gentle fantasies' of the 2-carbon phenethylamines. This is the works. There are visuals, and there are interpretive problems with knowing just where you really are. The place where nothing makes sense, and yet everything makes sense. I have just slept for a few hours, and now I am awake and it has been eighteen hours, and there is a lot still going on, although I have a relaxed, good feeling. Anyone who uses this had better have 24 hours at their disposal.
(with 2.4 mg) Here I am at the sixth hour, and I am still roaring along at a full plus three. I have established that this material is neither anti-erotic nor anorexic. The body is very comfortable, and so is the mind. There is an interesting aspect, perhaps peculiar only to this experiment and under these conditions. With my eyes closed the fantasy is a completely dark screen, lovely and seductive, subtle, and yet light must be deliberately brought in. This is not in any way negative for being in the dark, but is just unusual. I will have to try this in the daylight next time, to see what the eyes-closed brings to the mind-screen. At 24 hours, I have found that my sleep was not too great. My dreams were tight, and I kept defending against trouble; the nervous system was too alert. I was in a good humor, though, and I still am. This is excellent stuff, but start early in the day.
EXTENSIONS AND COMMENTARY: It is clear that the three halo-amphetamine derivatives, DOI, DOB and DOC, are all pretty much of the same potency. And all of them very long lived. The difference between the various halogen atoms was brought up under the 2C-C discussion. DOC is clearly a long-lasting, dyed-in-the-wool psychedelic.
In the making of this, by the procedures that have been followed in Canada, there are two chemical intermediates which might, some day, be looked at as potential psychedelics under their own colors. Reduction of the compound that is called DON in this Book II (2,5-dimethoxy-4-nitroamphetamine hydrochloride) with Pd/charcoal and hydrogen, gives the 4-amino derivative. This is 2,5-dimethoxy-4-aminoamphetamine dihydrochloride, DOA, which melts at 248-250 °C. And the reduction of an oxime intermediate gives rise to the acetamido analogue, 2,5-dimethoxy-4-acetamidoamphetamine hydrochloride, DOAA, with a mp of 249-250 °C. Neither compound has been tasted, but someday this omission will be corrected. DOA and DOAA have a sinister ring to them, however, and some changes of terminology might be needed. DOA, in the coroner's vocabulary, means Dead-On-Arrival. But then, AMA (the American Medical Association) just happens to also mean (in the jargon of emergency medicine) Against-Medical-Advice. Everything averages out, somehow. Remember that the amyl homolog (amyl at the 4-position) follows the 4-letter convention of all of the DOM homo-logues, and has the code name of DOAM. Thus, DOA, amino; DOAA, acetamido, and DOAM, amyl.
One must learn to keep one's sense of humor. The immortal humorist Wavy Gravy once said, "If you can't laugh at life, it just isn't funny anymore." The code name of this compound, 2,5-dimethoxy-4-chloroamphetamine is, after, all, DOC. This should certainly appeal to some physicians.
| [ |
[Main Index] | [Forward |

