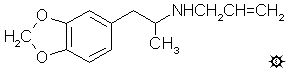
#101 MDAL
N-ALLYL-MDA; 3,4-METHYLENEDIOXY-N- ALLYLAMPHETAMINE
SYNTHESIS: A total of about 20 mL allylamine was introduced under the
surface of 20 mL concentrated HCl, and the mixture stripped of
volatiles under vacuum The resulting 24 g of wet material did not
yield any crystals with either acetone or Et2O. This was dissolved in
75 mL MeOH, treated with 4.45 g 3,4-methylenedioxy-phenylacetone (see
under MDMA for its preparation), and finally with 1.1 g sodium
cyanoborohydride. Concentrated HCl was added as needed over the
course of 5 days to keep the pH constant at about 6. The reaction
mixture was then added to a large amount of H2O, acidified with HCl,
and extracted with 3x100 mL CH2Cl2. The aqueous phase was made basic
with 25% NaOH, and extracted with 3x100 mL CH2Cl2. Evaporation of the
solvent from these extracts yielded 3.6 g of an amber oil which, on
distillation at 90-95 °C at 0.2 mm/Hg, yielded 2.6 g of an off-white
oil. This was dissolved in 10 mL IPA, neutralized with about 25 drops
of concentrated HCl, and the resulting clear but viscous solution was
diluted with Et2O until crystals formed. These were removed by
filtration, washed with IPA/Et2O (1:1), then with Et2O, and air dried
to constant weight. There was thus obtained 2.5 g of
3,4-methylenedioxy-N-allylamphetamine hydrochloride (MDAL) with a mp
of 174-176 °C and a proton NMR spectrum that showed that the allyl
group was intact. Anal. (C13H18ClNO2) N.
DOSAGE: greater than 180 mg.
DURATION: unknown.
EXTENSIONS AND COMMENTARY: Here is another inactive probe, like MDPR,
that could possibly serve as a primer to LSD. The three carbon chain
on the nitrogen seen with MDPR is almost identical to the three carbon
chain on the nitrogen atom of MDAL. And yet, where an "inactive"
level of 180 milligrams of MDPR is a rather fantastic enhancer of LSD
action, the same weight of this compound not only does not enhance,
but actually seems to somewhat antagonize the action of LSD. All this
difference from just a couple of hydrogen atoms. Identical carbon
atoms, identical oxygen atoms, and an identical nitrogen atom. And
all in identical places. Simply C13H18ClNO2 rather than C13H20ClNO2.
So, apparently, almost identical is not good enough!