
#102 MDBU
N-BUTYL-MDA; 3,4-METHYLENEDIOXY-N-BUTYLAMPHETAMINE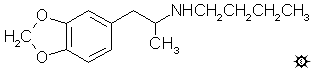
|
| [3D .mol structure] |
DOSAGE: greater than 40 mg.
DURATION: unknown.
EXTENSIONS AND COMMENTARY: Straight chain homologues on the nitrogen atom of MDA longer than two carbons are probably not active. This butyl compound provoked no interest, and although the longer chain counterparts were made by the general sodium cyanoborohydride method (see under MDBZ), they were not tasted. All mouse assays that compared this homologous series showed a consistent decrease in action (anesthetic potency and motor activity) as the alkyl chain on the nitrogen atoms was lengthened.
This synthetic procedure, using the hydrochloride salt of the amine and sodium cyanoborohydride in methanol, seems to be quite general for ketone compounds related to 3,4-methylenedioxyphenylacetone. Not only were most of the MD-group of compounds discussed here made in this manner, but the use of phenylacetone (phenyl-2-propanone, P-2-P) itself appears to be equally effective. The reaction of butylamine hydrochloride in methanol, with phenyl-2-propanone and sodium cyanoborohydride at pH of 6, after distillation at 70-75 °C at 0.3 mm/Hg, produced N-butylamphetamine hydrochloride (23.4 g from 16.3 g P-2-P). And, in the same manner with ethylamine hydrochloride there was produced N-ethylamphetamine (22.4 g from 22.1 g P-2-P) and with methylamine hydrochloride there was produced N-methylamphetamine hydrochloride (24.6 g from 26.8 g P-2-P). The reaction with simple ammonia (as ammonium acetate) gives consistently poor yields in these reactions.
| [ |
[Main Index] | [Forward |

